Carbamoyl radical-mediated synthesis and semipinacol rearrangement of β-lactam diols
- PMID: 24711140
- PMCID: PMC4320754
- DOI: 10.1002/chem.201304982
Carbamoyl radical-mediated synthesis and semipinacol rearrangement of β-lactam diols
Abstract
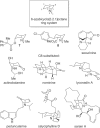
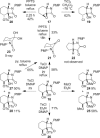
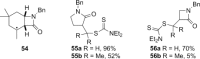
In an approach to the biologically important 6-azabicyclo[3.2.1]octane ring system, the scope of the tandem 4-exo-trig carbamoyl radical cyclization-dithiocarbamate group transfer reaction to ring-fused β-lactams is evaluated. β-Lactams fused to five-, six-, and seven-membered rings are prepared in good to excellent yield, and with moderate to complete control at the newly formed dithiocarbamate stereocentre. No cyclization is observed with an additional methyl substituent on the terminus of the double bond. Elimination of the dithiocarbamate group gives α,β- or β,γ-unsaturated lactams depending on both the methodology employed (base-mediated or thermal) and the nature of the carbocycle fused to the β-lactam. Fused β-lactam diols, obtained from catalytic OsO4-mediated dihydroxylation of α,β-unsaturated β-lactams, undergo semipinacol rearrangement via the corresponding cyclic sulfite or phosphorane to give keto-bridged bicyclic amides by exclusive N-acyl group migration. A monocyclic β-lactam diol undergoes Appel reaction at a primary alcohol in preference to semipinacol rearrangement. Preliminary investigations into the chemo- and stereoselective manipulation of the two carbonyl groups present in a representative 7,8-dioxo-6-azabicyclo[3.2.1]octane rearrangement product are also reported.
Keywords: cyclization; fused-ring systems; nitrogen heterocycles; ring expansion; strained molecules.
© 2014 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures















References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

