Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module
- PMID: 24711162
- PMCID: PMC4095802
- DOI: 10.1002/cncr.28672
Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: a prospective analysis using the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module
Abstract
Background: A prospective longitudinal study to profile patient-reported symptoms during radiotherapy (RT) or concurrent chemoradiotherapy (CCRT) for head and neck cancer was performed. The goals were to understand the onset and trajectory of specific symptoms and their severity, identify clusters, and facilitate symptom interventions and clinical trial design.
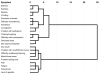
Methods: Participants in this questionnaire-based study received RT or CCRT. They completed the University of Texas MD Anderson Cancer Center Symptom Inventory-Head and Neck Module before and weekly during treatment. Symptom scores were compared between treatment groups, and hierarchical cluster analysis was used to depict clustering of symptoms at treatment end. Variables believed to predict symptom severity were assessed using a multivariate mixed model.
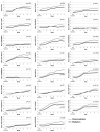
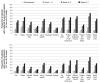
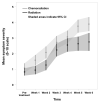
Results: Among the 149 patients studied, the majority (47%) had oropharyngeal tumors, and nearly one-half received CCRT. Overall symptom severity (P < .001) and symptom interference (P < .0001) became progressively more severe and were more severe for those receiving CCRT. On multivariate analysis, baseline Eastern Cooperative Oncology Group performance status (P < .001) and receipt of CCRT (P < .04) correlated with higher symptom severity. Fatigue, drowsiness, lack of appetite, problem with mouth/throat mucus, and problem tasting food were more severe for those receiving CCRT. Both local and systemic symptom clusters were identified.
Conclusions: The findings from this prospective longitudinal study identified a pattern of local and systemic symptoms, symptom clusters, and symptom interference that was temporally distinct and marked by increased magnitude and a shift in individual symptom rank order during the treatment course. These inform clinicians about symptom intervention needs, and are a benchmark for future symptom intervention clinical trials.
Keywords: chemoradiotherapy; head and neck cancer; patient-reported outcomes; radiotherapy; symptoms.
© 2014 American Cancer Society.
Figures
Comment in
-
Treatment deintensification and symptom burden in patients with human papillomavirus-associated head and neck cancer.Cancer. 2015 Apr 1;121(7):1147-8. doi: 10.1002/cncr.29152. Epub 2014 Nov 25. Cancer. 2015. PMID: 25424190 No abstract available.
References
-
- Cleeland CS, Sloan JA. Assessing the Symptoms of Cancer Using Patient-Reported Outcomes (ASCPRO): searching for standards. J Pain Symptom Manage. 2010;39(6):1077–1085. - PubMed
-
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. - PubMed
-
- NIH State-of-the-Science Statement on symptom management in cancer: pain, depression, and fatigue. NIH Consens State Sci Statements. 2002;19(4):1–29. - PubMed
-
- Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68(3):654–661. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

