HSCARG, a novel regulator of H2A ubiquitination by downregulating PRC1 ubiquitin E3 ligase activity, is essential for cell proliferation
- PMID: 24711370
- PMCID: PMC4027218
- DOI: 10.1093/nar/gku230
HSCARG, a novel regulator of H2A ubiquitination by downregulating PRC1 ubiquitin E3 ligase activity, is essential for cell proliferation
Abstract
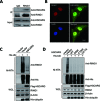
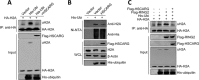
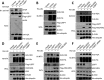
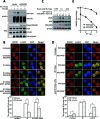
Histone H2A ubiquitination plays critical roles in transcriptional repression and deoxyribonucleic acid (DNA) damage response. More attention has been focused on ubiquitin E3 ligases of H2A, however, less is known about the negative regulators of H2A ubiquitination. Here we identified HSCARG as a new negative regulatory protein for H2A ubiquitination and found a possible link between regulator of H2A ubiquitination and cell cycle. Mechanistically, HSCARG interacts with polycomb repressive complex 1 (PRC1) and deubiquitinase USP7 and inhibits PRC1 ubiquitination in a USP7-dependent manner. As ubiquitination of PRC1 is critical for its E3 ligase activity toward H2A, HSCARG and USP7 are further shown to decrease the level of H2A ubiquitination. Moreover, we demonstrated that HSCARG is involved in DNA damage response through affecting the level of H2A ubiquitination and localization of RAP80 at lesion points. Knockout of HSCARG results in persistent activation of checkpoint signaling and leads to cell cycle arrest. This study unravels a novel mechanism for the regulation of H2A ubiquitination and elucidates how regulators of H2A ubiquitination affect cell cycle.
© The Author(s) 2014. The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
-
- Goldknopf I.L., Taylor C.W., Baum R.M., Yeoman L.C., Olson M.O., Prestayko A.W., Busch H. Isolation and characterization of protein A24, a ‘histone-like’ non-histone chromosomal protein. J. Biol. Chem. 1975;250:7182–7187. - PubMed
-
- Wang H., Wang L., Erdjument-Bromage H., Vidal M., Tempst P., Jones R.S., Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. - PubMed
-
- Cao R., Tsukada Y., Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell. 2005;20:845–854. - PubMed
-
- Mailand N., Bekker-Jensen S., Faustrup H., Melander F., Bartek J., Lukas C., Lukas J. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

