Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation
- PMID: 24711413
- PMCID: PMC4000844
- DOI: 10.1073/pnas.1404354111
Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation
Abstract
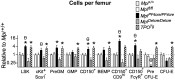
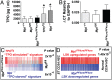
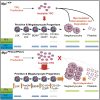
Thrombopoietin (TPO) acting via its receptor, the cellular homologue of the myeloproliferative leukemia virus oncogene (Mpl), is the major cytokine regulator of platelet number. To precisely define the role of specific hematopoietic cells in TPO-dependent hematopoiesis, we generated mice that express the Mpl receptor normally on stem/progenitor cells but lack expression on megakaryocytes and platelets (Mpl(PF4cre/PF4cre)). Mpl(PF4cre/PF4cre) mice displayed profound megakaryocytosis and thrombocytosis with a remarkable expansion of megakaryocyte-committed and multipotential progenitor cells, the latter displaying biological responses and a gene expression signature indicative of chronic TPO overstimulation as the underlying causative mechanism, despite a normal circulating TPO level. Thus, TPO signaling in megakaryocytes is dispensable for platelet production; its key role in control of platelet number is via generation and stimulation of the bipotential megakaryocyte precursors. Nevertheless, Mpl expression on megakaryocytes and platelets is essential to prevent megakaryocytosis and myeloproliferation by restricting the amount of TPO available to stimulate the production of megakaryocytes from the progenitor cell pool.
Keywords: bone marrow; essential thrombocythemia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
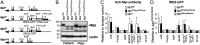

References
-
- Kuter DJ. Thrombopoietin Mimetics. In: Michelson AD, editor. Platelets. London: Academic; 2013. pp. 1217–1242.
-
- Qian S, Fu F, Li W, Chen Q, de Sauvage FJ. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. 1998;92(6):2189–2191. - PubMed
-
- Tiedt R, et al. Pronounced thrombocytosis in transgenic mice expressing reduced levels of Mpl in platelets and terminally differentiated megakaryocytes. Blood. 2009;113(8):1768–1777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

