Sugar demand, not auxin, is the initial regulator of apical dominance
- PMID: 24711430
- PMCID: PMC4000805
- DOI: 10.1073/pnas.1322045111
Sugar demand, not auxin, is the initial regulator of apical dominance
Abstract
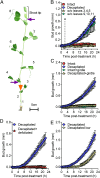
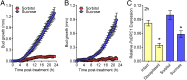
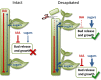
For almost a century the plant hormone auxin has been central to theories on apical dominance, whereby the growing shoot tip suppresses the growth of the axillary buds below. According to the classic model, the auxin indole-3-acetic acid is produced in the shoot tip and transported down the stem, where it inhibits bud growth. We report here that the initiation of bud growth after shoot tip loss cannot be dependent on apical auxin supply because we observe bud release up to 24 h before changes in auxin content in the adjacent stem. After the loss of the shoot tip, sugars are rapidly redistributed over large distances and accumulate in axillary buds within a timeframe that correlates with bud release. Moreover, artificially increasing sucrose levels in plants represses the expression of BRANCHED1 (BRC1), the key transcriptional regulator responsible for maintaining bud dormancy, and results in rapid bud release. An enhancement in sugar supply is both necessary and sufficient for suppressed buds to be released from apical dominance. Our data support a theory of apical dominance whereby the shoot tip's strong demand for sugars inhibits axillary bud outgrowth by limiting the amount of sugar translocated to those buds.
Keywords: decapitation; girdling; long-distance signaling; shoot branching; sink demand.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Thimann KV, Skoog F, Kerckhoff WG. On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc R Soc Lond B Biol Sci. 1934;114(798):317–339.
-
- Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol. 2011;12(4):211–221. - PubMed
-
- Gomez-Roldan V, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455(7210):189–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

