Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation
- PMID: 24711432
- PMCID: PMC4000837
- DOI: 10.1073/pnas.1402251111
Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation
Abstract
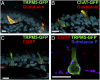
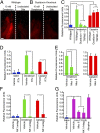
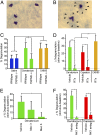
Solitary chemosensory cells (SCCs) of the nasal cavity are specialized epithelial chemosensors that respond to irritants through the canonical taste transduction cascade involving Gα-gustducin and transient receptor potential melastatin 5. When stimulated, SCCs trigger peptidergic nociceptive (or pain) nerve fibers, causing an alteration of the respiratory rate indicative of trigeminal activation. Direct chemical excitation of trigeminal pain fibers by capsaicin evokes neurogenic inflammation in the surrounding epithelium. In the current study, we test whether activation of nasal SCCs can trigger similar local inflammatory responses, specifically mast cell degranulation and plasma leakage. The prototypical bitter compound, denatonium, a well-established activator of SCCs, caused significant inflammatory responses in WT mice but not mice with a genetic deletion of elements of the canonical taste transduction cascade, showing that activation of taste signaling components is sufficient to trigger local inflammation. Chemical ablation of peptidergic trigeminal fibers prevented the SCC-induced nasal inflammation, indicating that SCCs evoke inflammation only by neural activity and not by release of local inflammatory mediators. Additionally, blocking nicotinic, but not muscarinic, acetylcholine receptors prevents SCC-mediated neurogenic inflammation for both denatonium and the bacterial signaling molecule 3-oxo-C12-homoserine lactone, showing the necessity for cholinergic transmission. Finally, we show that the neurokinin 1 receptor for substance P is required for SCC-mediated inflammation, suggesting that release of substance P from nerve fibers triggers the inflammatory events. Taken together, these results show that SCCs use cholinergic neurotransmission to trigger peptidergic trigeminal nociceptors, which link SCCs to the neurogenic inflammatory pathway.
Keywords: airway irritation; chemesthesis; innate immunity; quorum sensing; rhinitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Silver W, Roe P, Saunders CJ. Functional neuroanatomy of the upper airway in experimental animals. In: Morris JB, Shusterman D, editors. Toxicology of the Nose and Upper Airways. New York: Informa Healthcare; 2010. pp. 45–64.
-
- Silver WL, Finger TE. The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann N Y Acad Sci. 2009;1170:202–205. - PubMed
-
- Bryant BP, Silver W. Chemesthesis: The common chemical sense. In: Finger TE, Silver W, Restrepo D, editors. Neurobiology of Taste and Smell. 2nd Ed. New York: Wiley-Liss; 2000. pp. 73–100.
-
- Kobayashi K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493(4):596–606. - PubMed
-
- Finger TE, St Jeor VL, Kinnamon JC, Silver WL. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol. 1990;294(2):293–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

