Multidrug transport protein norM from vibrio cholerae simultaneously couples to sodium- and proton-motive force
- PMID: 24711447
- PMCID: PMC4031518
- DOI: 10.1074/jbc.M113.546770
Multidrug transport protein norM from vibrio cholerae simultaneously couples to sodium- and proton-motive force
Abstract
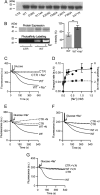
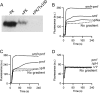
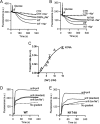
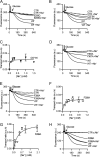
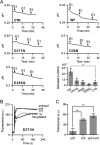
Membrane transporters belonging to the multidrug and toxic compound extrusion family mediate the efflux of unrelated pharmaceuticals from the interior of the cell in organisms ranging from bacteria to human. These proteins are thought to fall into two classes that couple substrate efflux to the influx of either Na(+) or H(+). We studied the energetics of drug extrusion by NorM from Vibrio cholerae in proteoliposomes in which purified NorM protein was functionally reconstituted in an inside-out orientation. We establish that NorM simultaneously couples to the sodium-motive force and proton-motive force, and biochemically identify protein regions and residues that play important roles in Na(+) or H(+) binding. As the positions of protons are not available in current medium and high-resolution crystal structures of multidrug and toxic compound extrusion transporters, our findings add a previously unrecognized parameter to mechanistic models based of these structures.
Keywords: Bioenergetics; Enzyme Mechanisms; Membrane Proteins; Membrane Transport; Multidrug Transporters.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
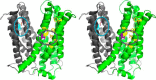
References
-
- Omote H., Hiasa M., Matsumoto T., Otsuka M., Moriyama Y. (2006) The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol. Sci. 27, 587–593 - PubMed
-
- Kuroda T., Tsuchiya T. (2009) Multidrug efflux transporters in the MATE family. Biochim. Biophys. Acta 1794, 763–768 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

