Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation
- PMID: 24711499
- PMCID: PMC3987140
- DOI: 10.1083/jcb.201310114
Coordinated binding of Vps4 to ESCRT-III drives membrane neck constriction during MVB vesicle formation
Abstract
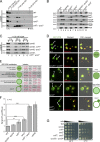
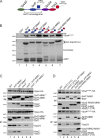
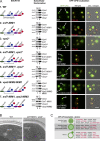
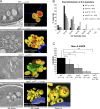
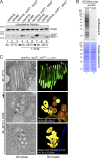
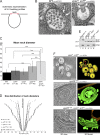
Five endosomal sorting complexes required for transport (ESCRTs) mediate the degradation of ubiquitinated membrane proteins via multivesicular bodies (MVBs) in lysosomes. ESCRT-0, -I, and -II interact with cargo on endosomes. ESCRT-II also initiates the assembly of a ringlike ESCRT-III filament consisting of Vps20, Snf7, Vps24, and Vps2. The AAA-adenosine triphosphatase Vps4 disassembles and recycles the ESCRT-III complex, thereby terminating the ESCRT pathway. A mechanistic role for Vps4 in intraluminal vesicle (ILV) formation has been unclear. By combining yeast genetics, biochemistry, and electron tomography, we find that ESCRT-III assembly on endosomes is required to induce or stabilize the necks of growing MVB ILVs. Yet, ESCRT-III alone is not sufficient to complete ILV biogenesis. Rather, binding of Vps4 to ESCRT-III, coordinated by interactions with Vps2 and Snf7, is coupled to membrane neck constriction during ILV formation. Thus, Vps4 not only recycles ESCRT-III subunits but also cooperates with ESCRT-III to drive distinct membrane-remodeling steps, which lead to efficient membrane scission at the end of ILV biogenesis in vivo.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

