A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells
- PMID: 24711500
- PMCID: PMC3987145
- DOI: 10.1083/jcb.201311104
A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells
Abstract
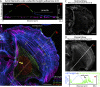
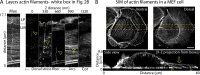
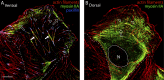
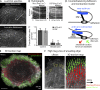
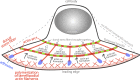
How adherent and contractile systems coordinate to promote cell shape changes is unclear. Here, we define a counterbalanced adhesion/contraction model for cell shape control. Live-cell microscopy data showed a crucial role for a contractile meshwork at the top of the cell, which is composed of actin arcs and myosin IIA filaments. The contractile actin meshwork is organized like muscle sarcomeres, with repeating myosin II filaments separated by the actin bundling protein α-actinin, and is mechanically coupled to noncontractile dorsal actin fibers that run from top to bottom in the cell. When the meshwork contracts, it pulls the dorsal fibers away from the substrate. This pulling force is counterbalanced by the dorsal fibers' attachment to focal adhesions, causing the fibers to bend downward and flattening the cell. This model is likely to be relevant for understanding how cells configure themselves to complex surfaces, protrude into tight spaces, and generate three-dimensional forces on the growth substrate under both healthy and diseased conditions.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

