Quality of life in MAP.3 (Mammary Prevention 3): a randomized, placebo-controlled trial evaluating exemestane for prevention of breast cancer
- PMID: 24711552
- PMCID: PMC4879707
- DOI: 10.1200/JCO.2013.51.2483
Quality of life in MAP.3 (Mammary Prevention 3): a randomized, placebo-controlled trial evaluating exemestane for prevention of breast cancer
Abstract
Purpose: Exemestane, a steroidal aromatase inhibitor, reduced invasive breast cancer incidence by 65% among 4,560 postmenopausal women randomly assigned to exemestane (25 mg per day) compared with placebo in the National Cancer Institute of Canada (NCIC) Clinical Trials Group MAP.3 (Mammary Prevention 3) trial, but effects on quality of life (QOL) were not fully described.
Patients and methods: Menopause-specific and health-related QOL were assessed by using the four Menopause-Specific Quality of Life Questionnaire (MENQOL) domains and the eight Medical Outcomes Study Short Form Health Survey (SF-36) scales at baseline, 6 months, and yearly thereafter. MENQOL questionnaire completion was high (88% to 98%) in both groups at each follow-up visit. Change scores for each MENQOL and SF-36 scale, calculated at each assessment time relative to baseline, were compared by using the Wilcoxon rank-sum test. Clinically important worsened QOL was defined as a MENQOL change score increase of more than 0.5 (of 8) points and an SF-36 change score decrease of more than 5 (of 100) points from baseline.
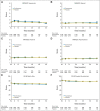
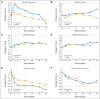
Results: Exemestane had small negative effects on women's self-reported vasomotor symptoms, sexual symptoms, and pain, which occurred mainly in the first 6 months to 2 years after random assignment. However, these changes represented only a small excess number of women being given exemestane with clinically important worsening of QOL at one time or another; specifically, 8% more in the vasomotor domain and 4% more each in the sexual domain and for pain. No other between-group differences were observed. Overall, slightly more women in the exemestane arm (32%) than in the placebo arm (28%) discontinued assigned treatment.
Conclusion: Exemestane given for prevention has limited negative impact on menopause-specific and health-related QOL in healthy postmenopausal women at risk for breast cancer.
Trial registration: ClinicalTrials.gov NCT00083174.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Reply to P. Niravath et Al.J Clin Oncol. 2014 Nov 20;32(33):3780-1. doi: 10.1200/JCO.2014.57.9235. Epub 2014 Aug 25. J Clin Oncol. 2014. PMID: 25154825 No abstract available.
-
Aromatase inhibitor adverse effects: are we sweeping them under the rug?J Clin Oncol. 2014 Nov 20;32(33):3779. doi: 10.1200/JCO.2014.56.9640. Epub 2014 Aug 25. J Clin Oncol. 2014. PMID: 25154832 No abstract available.
References
-
- Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:2942–2962. - PubMed
-
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. - PubMed
-
- Ganz PA. Breast cancer, menopause, and long-term survivorship: Critical issues for the 21st century. Am J Med. 2005;118:136–141. - PubMed
-
- Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: Understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. - PubMed
-
- Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–1846. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

