Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection
- PMID: 24711580
- PMCID: PMC4010906
- DOI: 10.1084/jem.20131556
Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection
Abstract
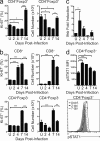
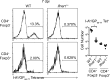
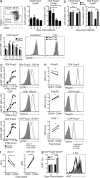
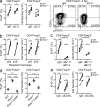
Regulatory T (T reg) cells play an essential role in preventing autoimmunity but can also impair clearance of foreign pathogens. Paradoxically, signals known to promote T reg cell function are abundant during infection and could inappropriately enhance T reg cell activity. How T reg cell function is restrained during infection to allow the generation of effective antiviral responses remains largely unclear. We demonstrate that the potent antiviral type I interferons (IFNs) directly inhibit co-stimulation-dependent T reg cell activation and proliferation, both in vitro and in vivo during acute infection with lymphocytic choriomeningitis virus (LCMV). Loss of the type I IFN receptor specifically in T reg cells results in functional impairment of virus-specific CD8(+) and CD4(+) T cells and inefficient viral clearance. Together, these data demonstrate that inhibition of T reg cells by IFNs is necessary for the generation of optimal antiviral T cell responses during acute LCMV infection.
Figures


Similar articles
-
Functional limitations of plasmacytoid dendritic cells limit type I interferon, T cell responses and virus control in early life.PLoS One. 2013 Dec 23;8(12):e85302. doi: 10.1371/journal.pone.0085302. eCollection 2013. PLoS One. 2013. PMID: 24376875 Free PMC article.
-
Type I interferon signaling attenuates regulatory T cell function in viral infection and in the tumor microenvironment.PLoS Pathog. 2018 Apr 19;14(4):e1006985. doi: 10.1371/journal.ppat.1006985. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29672594 Free PMC article.
-
Role of PKR and Type I IFNs in viral control during primary and secondary infection.PLoS Pathog. 2010 Jun 24;6(6):e1000966. doi: 10.1371/journal.ppat.1000966. PLoS Pathog. 2010. PMID: 20585572 Free PMC article.
-
Immune Exhaustion: Past Lessons and New Insights from Lymphocytic Choriomeningitis Virus.Viruses. 2019 Feb 13;11(2):156. doi: 10.3390/v11020156. Viruses. 2019. PMID: 30781904 Free PMC article. Review.
-
Complexities of Type I Interferon Biology: Lessons from LCMV.Viruses. 2019 Feb 20;11(2):172. doi: 10.3390/v11020172. Viruses. 2019. PMID: 30791575 Free PMC article. Review.
Cited by
-
The myeloid cell type I IFN system promotes antitumor immunity over pro-tumoral inflammation in cancer T-cell therapy.Clin Transl Immunology. 2021 Apr 29;10(4):e1276. doi: 10.1002/cti2.1276. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33968406 Free PMC article.
-
In vitro antiproliferation activity of Typhonium flagelliforme leaves ethanol extract and its combination with canine interferons on several tumor-derived cell lines.Vet World. 2020 May;13(5):931-939. doi: 10.14202/vetworld.2020.931-939. Epub 2020 May 19. Vet World. 2020. PMID: 32636590 Free PMC article.
-
Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells.Nat Immunol. 2015 Aug;16(8):871-9. doi: 10.1038/ni.3224. Epub 2015 Jul 6. Nat Immunol. 2015. PMID: 26147684 Free PMC article.
-
The Interactions Between Cancer Stem Cells and the Innate Interferon Signaling Pathway.Front Immunol. 2020 Mar 31;11:526. doi: 10.3389/fimmu.2020.00526. eCollection 2020. Front Immunol. 2020. PMID: 32296435 Free PMC article. Review.
-
The Role of Interferon in Persistent Viral Infection: Insights from Murine Norovirus.Trends Microbiol. 2018 Jun;26(6):510-524. doi: 10.1016/j.tim.2017.10.010. Epub 2017 Nov 17. Trends Microbiol. 2018. PMID: 29157967 Free PMC article. Review.
References
-
- Ahmed R., Salmi A., Butler L.D., Chiller J.M., Oldstone M.B. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540 10.1084/jem.160.2.521 - DOI - PMC - PubMed
-
- Benson A., Murray S., Divakar P., Burnaevskiy N., Pifer R., Forman J., Yarovinsky F. 2012. Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J. Immunol. 188:800–810 10.4049/jimmunol.1100769 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

