Utility of Urinary Biomarkers in Predicting Loss of Residual Renal Function: The balANZ Trial
- PMID: 24711637
- PMCID: PMC4406311
- DOI: 10.3747/pdi.2013.00170
Utility of Urinary Biomarkers in Predicting Loss of Residual Renal Function: The balANZ Trial
Abstract
Background: The ability of urinary biomarkers to predict residual renal function (RRF) decline in peritoneal dialysis (PD) patients has not been defined. The present study aimed to explore the utility of established biomarkers from kidney injury models for predicting loss of RRF in incident PD patients, and to evaluate the impact on RRF of using neutral-pH PD solution low in glucose degradation products.
Methods: The study included 50 randomly selected participants from the balANZ trial who had completed 24 months of follow-up. A change in glomerular filtration rate (GFR) was used as the primary clinical outcome measure. In a mixed-effects general linear model, baseline measurements of 18 novel urinary biomarkers and albumin were used to predict GFR change. The model was further used to evaluate the impact of biocompatible PD solution on RRF, adjusted for each biomarker.
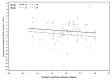
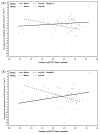
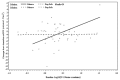
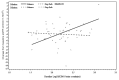
Results: Baseline albuminuria was not a useful predictor of change in RRF in PD patients (p = 0.84). Only clusterin was a significant predictor of GFR decline in the whole population (p = 0.04, adjusted for baseline GFR and albuminuria). However, the relationship was no longer apparent when albuminuria was removed from the model (p = 0.31). When the effect of the administered PD solutions was examined using a model adjusted for PD solution type, baseline albuminuria, and GFR, higher baseline urinary concentrations of trefoil factor 3 (TFF3, p = 0.02), kidney injury molecule 1 (KIM-1, p = 0.04), and interferon γ-induced protein 10 (IP-10, p = 0.03) were associated with more rapid decline of RRF in patients receiving conventional PD solution compared with biocompatible PD solution.
Conclusions: Higher urinary levels of kidney injury biomarkers (TFF3, KIM-1, IP-10) at baseline predicted significantly slower RRF decline in patients receiving biocompatible PD solutions. Findings from the present investigation should help to guide future studies to validate the utility of urinary biomarkers as tools to predict RRF decline in PD patients.
Keywords: Biocompatibility; biomarkers; glucose degradation products; kidney injury; residual renal function.
Copyright © 2015 International Society for Peritoneal Dialysis.
Figures
References
-
- Bargman JM, Thorpe KE, Churchill DN on behalf of the CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12:2158–62. - PubMed
-
- Justo P, Sanz AB, Egido J, Ortiz A. 3,4-Dideoxyglucosone-3-ene induces apoptosis in renal tubular epithelial cells. Diabetes 2005; 54:2424–9. - PubMed
-
- Cho Y, Johnson DW, Badve SV, Craig JC, Strippoli GF, Wiggins K. The impact of neutral-pH peritoneal dialysates with reduced glucose degradation products on clinical outcomes in peritoneal dialysis patients. Kidney Int 2013; 84:969–79. - PubMed
-
- Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Perit Dial Int 2008; 28:174–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

