Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods
- PMID: 24711712
- PMCID: PMC3969256
- DOI: 10.2147/CMAR.S54726
Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods
Abstract
Malignant gliomas consist of glioblastomas, anaplastic astrocytomas, anaplastic oligodendrogliomas and anaplastic oligoastrocytomas, and some less common tumors such as anaplastic ependymomas and anaplastic gangliogliomas. Malignant gliomas have high morbidity and mortality. Even with optimal treatment, median survival is only 12-15 months for glioblastomas and 2-5 years for anaplastic gliomas. However, recent advances in imaging and quantitative analysis of image data have led to earlier diagnosis of tumors and tumor response to therapy, providing oncologists with a greater time window for therapy management. In addition, improved understanding of tumor biology, genetics, and resistance mechanisms has enhanced surgical techniques, chemotherapy methods, and radiotherapy administration. After proper diagnosis and institution of appropriate therapy, there is now a vital need for quantitative methods that can sensitively detect malignant glioma response to therapy at early follow-up times, when changes in management of nonresponders can have its greatest effect. Currently, response is largely evaluated by measuring magnetic resonance contrast and size change, but this approach does not take into account the key biologic steps that precede tumor size reduction. Molecular imaging is ideally suited to measuring early response by quantifying cellular metabolism, proliferation, and apoptosis, activities altered early in treatment. We expect that successful integration of quantitative imaging biomarker assessment into the early phase of clinical trials could provide a novel approach for testing new therapies, and importantly, for facilitating patient management, sparing patients from weeks or months of toxicity and ineffective treatment. This review will present an overview of epidemiology, molecular pathogenesis and current advances in diagnoses, and management of malignant gliomas.
Keywords: FLT; MRI; PET; early therapy response assessment; glioblastoma multiforme; malignant gliomas; quantitative molecular imaging.
Figures
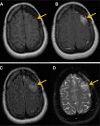
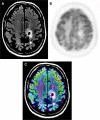
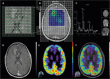
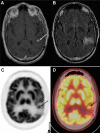
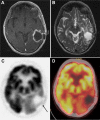
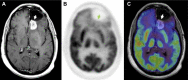
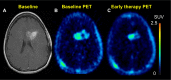
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. - PubMed
-
- Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225. discussion 226–229. - PubMed
-
- Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007;25(4):867–890. vii. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

