Microbially-accelerated consolidation of oil sands tailings. Pathway II: solid phase biogeochemistry
- PMID: 24711806
- PMCID: PMC3968759
- DOI: 10.3389/fmicb.2014.00107
Microbially-accelerated consolidation of oil sands tailings. Pathway II: solid phase biogeochemistry
Abstract
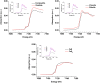
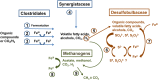
Consolidation of clay particles in aqueous tailings suspensions is a major obstacle to effective management of oil sands tailings ponds in northern Alberta, Canada. We have observed that microorganisms indigenous to the tailings ponds accelerate consolidation of mature fine tailings (MFT) during active metabolism by using two biogeochemical pathways. In Pathway I, microbes alter porewater chemistry to indirectly increase consolidation of MFT. Here, we describe Pathway II comprising significant, direct and complementary biogeochemical reactions with MFT mineral surfaces. An anaerobic microbial community comprising Bacteria (predominantly Clostridiales, Synergistaceae, and Desulfobulbaceae) and Archaea (Methanolinea/Methanoregula and Methanosaeta) transformed Fe(III) minerals in MFT to amorphous Fe(II) minerals during methanogenic metabolism of an added organic substrate. Synchrotron analyses suggested that ferrihydrite (5Fe2O3. 9H2O) and goethite (α-FeOOH) were the dominant Fe(III) minerals in MFT. The formation of amorphous iron sulfide (FeS) and possibly green rust entrapped and masked electronegative clay surfaces in amended MFT. Both Pathways I and II reduced the surface charge potential (repulsive forces) of the clay particles in MFT, which aided aggregation of clays and formation of networks of pores, as visualized using cryo-scanning electron microscopy (SEM). These reactions facilitated the egress of porewater from MFT and increased consolidation of tailings solids. These results have large-scale implications for management and reclamation of oil sands tailings ponds, a burgeoning environmental issue for the public and government regulators.
Keywords: FeIII reduction; aggregation of clay particles; consolidation; formation of FeII minerals; methanogenesis; oil sands tailings.
Figures





References
-
- Ahern C. R., McElnea A. E., Sullivan L. A. (1998). Acid Sulfate Soils Laboratory Methods Guidelines. Wollongbar, NSW: Acid Sulfate Soil Management Advisory Committee
-
- Allen H. E., Fu G., Deng B. (1993). Analysis of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ. Toxico. Chem. 12, 1441–1453 10.1002/etc.5620120812 - DOI
-
- Borggaard O. K. (1988). Phase identification by selective dissolution techniques, in Iron in Soils and Clay Minerals, eds Stucki J. W., Goodman B. A., Schwettmann U. (Dordrecht: Reidel; ), 83–98
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

