The dopaminergic system in autoimmune diseases
- PMID: 24711809
- PMCID: PMC3968755
- DOI: 10.3389/fimmu.2014.00117
The dopaminergic system in autoimmune diseases
Abstract
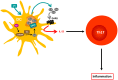
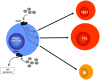
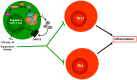
Bidirectional interactions between the immune and the nervous systems are of considerable interest both for deciphering their functioning and for designing novel therapeutic strategies. The past decade has brought a burst of insights into the molecular mechanisms involved in neuroimmune communications mediated by dopamine. Studies of dendritic cells (DCs) revealed that they express the whole machinery to synthesize and store dopamine, which may act in an autocrine manner to stimulate dopamine receptors (DARs). Depending on specific DARs stimulated on DCs and T cells, dopamine may differentially favor CD4(+) T cell differentiation into Th1 or Th17 inflammatory cells. Regulatory T cells can also release high amounts of dopamine that acts in an autocrine DAR-mediated manner to inhibit their suppressive activity. These dopaminergic regulations could represent a driving force during autoimmunity. Indeed, dopamine levels are altered in the brain of mouse models of multiple sclerosis (MS) and lupus, and in inflamed tissues of patients with inflammatory bowel diseases or rheumatoid arthritis (RA). The distorted expression of DARs in peripheral lymphocytes of lupus and MS patients also supports the importance of dopaminergic regulations in autoimmunity. Moreover, dopamine analogs had beneficial therapeutic effects in animal models, and in patients with lupus or RA. We propose models that may underlie key roles of dopamine and its receptors in autoimmune diseases.
Keywords: Crohn’s disease; Th17; dendritic cell; multiple sclerosis; regulatory T cell; rheumatoid arthritis; systemic lupus erythematosus; ulcerative colitis.
Figures
Similar articles
-
Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model.J Immunol. 2011 Mar 15;186(6):3745-52. doi: 10.4049/jimmunol.1002475. Epub 2011 Feb 9. J Immunol. 2011. PMID: 21307293
-
Dopamine and T cells: dopamine receptors and potent effects on T cells, dopamine production in T cells, and abnormalities in the dopaminergic system in T cells in autoimmune, neurological and psychiatric diseases.Acta Physiol (Oxf). 2016 Jan;216(1):42-89. doi: 10.1111/apha.12476. Epub 2015 Sep 24. Acta Physiol (Oxf). 2016. PMID: 25728499 Review.
-
Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity.J Immunol. 2012 Apr 1;188(7):3062-70. doi: 10.4049/jimmunol.1103096. Epub 2012 Feb 29. J Immunol. 2012. PMID: 22379034
-
Targeting the Dopaminergic System in Autoimmunity.J Neuroimmune Pharmacol. 2020 Mar;15(1):57-73. doi: 10.1007/s11481-019-09834-5. Epub 2019 Jan 19. J Neuroimmune Pharmacol. 2020. PMID: 30661214 Review.
-
Green tea EGCG, T cells, and T cell-mediated autoimmune diseases.Mol Aspects Med. 2012 Feb;33(1):107-18. doi: 10.1016/j.mam.2011.10.001. Epub 2011 Oct 14. Mol Aspects Med. 2012. PMID: 22020144 Review.
Cited by
-
The effects of dopamine receptor 2 expression on B cells on bone metabolism and TNF-α levels in rheumatoid arthritis.BMC Musculoskelet Disord. 2016 Aug 19;17:352. doi: 10.1186/s12891-016-1220-7. BMC Musculoskelet Disord. 2016. PMID: 27542839 Free PMC article.
-
Inflammation Effects on Motivation and Motor Activity: Role of Dopamine.Neuropsychopharmacology. 2017 Jan;42(1):216-241. doi: 10.1038/npp.2016.143. Epub 2016 Aug 2. Neuropsychopharmacology. 2017. PMID: 27480574 Free PMC article. Review.
-
Chemokinergic and Dopaminergic Signalling Collaborates through the Heteromer Formed by CCR9 and Dopamine Receptor D5 Increasing the Migratory Speed of Effector CD4+ T-Cells to Infiltrate the Colonic Mucosa.Int J Mol Sci. 2024 Sep 18;25(18):10022. doi: 10.3390/ijms251810022. Int J Mol Sci. 2024. PMID: 39337509 Free PMC article.
-
Role of the End-Point Mediators of Sympathoadrenal and Sympathoneural Stress Axes in the Pathogenesis of Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis.Front Endocrinol (Lausanne). 2020 Jan 14;10:921. doi: 10.3389/fendo.2019.00921. eCollection 2019. Front Endocrinol (Lausanne). 2020. PMID: 31993021 Free PMC article. Review.
-
Dual-Tasking in Multiple Sclerosis - Implications for a Cognitive Screening Instrument.Front Hum Neurosci. 2018 Jan 31;12:24. doi: 10.3389/fnhum.2018.00024. eCollection 2018. Front Hum Neurosci. 2018. PMID: 29445335 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

