Skeletal gene expression in the temporal region of the reptilian embryos: implications for the evolution of reptilian skull morphology
- PMID: 24711977
- PMCID: PMC3970585
- DOI: 10.1186/2193-1801-2-336
Skeletal gene expression in the temporal region of the reptilian embryos: implications for the evolution of reptilian skull morphology
Abstract
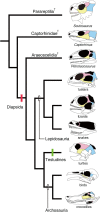
Reptiles have achieved highly diverse morphological and physiological traits that allow them to exploit various ecological niches and resources. Morphology of the temporal region of the reptilian skull is highly diverse and historically it has been treated as an important character for classifying reptiles and has helped us understand the ecology and physiology of each species. However, the developmental mechanism that generates diversity of reptilian skull morphology is poorly understood. We reveal a potential developmental basis that generates morphological diversity in the temporal region of the reptilian skull by performing a comparative analysis of gene expression in the embryos of reptile species with different skull morphology. By investigating genes known to regulate early osteoblast development, we find dorsoventrally broadened unique expression of the early osteoblast marker, Runx2, in the temporal region of the head of turtle embryos that do not form temporal fenestrae. We also observe that Msx2 is also uniquely expressed in the mesenchymal cells distributed at the temporal region of the head of turtle embryos. Furthermore, through comparison of gene expression pattern in the embryos of turtle, crocodile, and snake species, we find a possible correlation between the spatial patterns of Runx2 and Msx2 expression in cranial mesenchymal cells and skull morphology of each reptilian lineage. Regulatory modifications of Runx2 and Msx2 expression in osteogenic mesenchymal precursor cells are likely involved in generating morphological diversity in the temporal region of the reptilian skull.
Keywords: Development; Heterotopy; Morphology; Osteogenesis; Reptiles; Skull.
Figures







Similar articles
-
Dynamic evolutionary interplay between ontogenetic skull patterning and whole-head integration.Nat Ecol Evol. 2024 Mar;8(3):536-551. doi: 10.1038/s41559-023-02295-3. Epub 2024 Jan 10. Nat Ecol Evol. 2024. PMID: 38200368
-
Turtle skull development unveils a molecular basis for amniote cranial diversity.Sci Adv. 2023 Nov 17;9(46):eadi6765. doi: 10.1126/sciadv.adi6765. Epub 2023 Nov 15. Sci Adv. 2023. PMID: 37967181 Free PMC article.
-
Creating morphological diversity in reptilian temporal skull region: A review of potential developmental mechanisms.Evol Dev. 2023 Jan;25(1):15-31. doi: 10.1111/ede.12419. Epub 2022 Oct 17. Evol Dev. 2023. PMID: 36250751 Review.
-
Morphology of the temporal skull region in tetrapods: research history, functional explanations, and a new comprehensive classification scheme.Biol Rev Camb Philos Soc. 2021 Oct;96(5):2229-2257. doi: 10.1111/brv.12751. Epub 2021 May 31. Biol Rev Camb Philos Soc. 2021. PMID: 34056833
-
Efficient harvesting methods for early-stage snake and turtle embryos.Dev Growth Differ. 2016 Apr;58(3):241-9. doi: 10.1111/dgd.12278. Epub 2016 Apr 5. Dev Growth Differ. 2016. PMID: 27059539 Review.
Cited by
-
Dynamic evolutionary interplay between ontogenetic skull patterning and whole-head integration.Nat Ecol Evol. 2024 Mar;8(3):536-551. doi: 10.1038/s41559-023-02295-3. Epub 2024 Jan 10. Nat Ecol Evol. 2024. PMID: 38200368
-
Turtle skull development unveils a molecular basis for amniote cranial diversity.Sci Adv. 2023 Nov 17;9(46):eadi6765. doi: 10.1126/sciadv.adi6765. Epub 2023 Nov 15. Sci Adv. 2023. PMID: 37967181 Free PMC article.
-
Divergent palate morphology in turtles and birds correlates with differences in proliferation and BMP2 expression during embryonic development.J Exp Zool B Mol Dev Evol. 2014 Feb;322(2):73-85. doi: 10.1002/jez.b.22547. Epub 2013 Dec 9. J Exp Zool B Mol Dev Evol. 2014. PMID: 24323766 Free PMC article.
-
Palaeoneurological clues to the evolution of defining mammalian soft tissue traits.Sci Rep. 2016 May 9;6:25604. doi: 10.1038/srep25604. Sci Rep. 2016. PMID: 27157809 Free PMC article.
-
Creating diversity in mammalian facial morphology: a review of potential developmental mechanisms.Evodevo. 2018 Jun 14;9:15. doi: 10.1186/s13227-018-0103-4. eCollection 2018. Evodevo. 2018. PMID: 29946416 Free PMC article. Review.
References
-
- Benton MJ. Vertebrate palaeontology 3rd edition. Malden: Blackwell Publishing; 2005.
LinkOut - more resources
Full Text Sources
Other Literature Sources

