Sex- and season-dependent differences in telomere length and telomerase activity in the leaves of ash and willow
- PMID: 24711987
- PMCID: PMC3977023
- DOI: 10.1186/2193-1801-3-163
Sex- and season-dependent differences in telomere length and telomerase activity in the leaves of ash and willow
Abstract
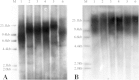
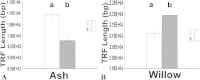
Telomeres and telomerase have important biological functions and can protect chromosome ends. In this study, sex- and season-dependent changes in telomere length and telomerase activity in ash and willow were analyzed. A statistical analysis showed that the telomere lengths of male and female trees differed significantly (P < 0.05). In ash, the telomere lengths of female trees were shorter than those of male trees. In willow, the telomere lengths of female trees were longer than those of male trees. During the annual developmental cycle, the telomere lengths of male and female ash and willow increased from April to May (P < 0.05), remained stable from May to August (P > 0.05), and decreased significantly in September and October (P < 0.05). Additionally, telomerase activities could be detected in both male and female ash and willow trees from April to October. Our results show that the telomere lengths changed according to season and sex in ash and willow. Telomere length did not have a direct positive correlation with telomerase activity.
Keywords: Ash; Season-specific; Sex-specific; Telomerase activity; Telomere length; Willow.
Figures



Similar articles
-
Season- and age-associated telomerase activity in Ginkgo biloba L.Mol Biol Rep. 2011 Mar;38(3):1799-805. doi: 10.1007/s11033-010-0295-8. Epub 2010 Sep 15. Mol Biol Rep. 2011. PMID: 20842436
-
Telomere length homeostasis.Chromosoma. 2006 Dec;115(6):413-25. doi: 10.1007/s00412-006-0067-3. Epub 2006 Jun 2. Chromosoma. 2006. PMID: 16741708 Review.
-
Change of season-specific telomere lengths in Ginkgo biloba L.Mol Biol Rep. 2010 Feb;37(2):819-24. doi: 10.1007/s11033-009-9627-y. Epub 2009 Jul 21. Mol Biol Rep. 2010. PMID: 19626460
-
Telomere length and telomerase activity in bladder and prostate cancer cell lines.Int J Urol. 1997 Jul;4(4):407-10. doi: 10.1111/j.1442-2042.1997.tb00216.x. Int J Urol. 1997. PMID: 9256332
-
[Maintaining telomere length].Postepy Hig Med Dosw (Online). 2013 Dec 17;67:1319-30. doi: 10.5604/17322693.1081034. Postepy Hig Med Dosw (Online). 2013. PMID: 24379272 Review. Polish.
Cited by
-
Telomere Length in Norway Spruce during Somatic Embryogenesis and Cryopreservation.Plants (Basel). 2021 Feb 23;10(2):416. doi: 10.3390/plants10020416. Plants (Basel). 2021. PMID: 33672393 Free PMC article.
-
Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up.Elife. 2020 Feb 11;9:e51507. doi: 10.7554/eLife.51507. Elife. 2020. PMID: 32041686 Free PMC article.
References
-
- Aronen T, Ryynänen L. Variation in telomeric repeats of Scots pine (Pinus sylvestris L.) Tree Genet Genomes. 2012;8:267–275. doi: 10.1007/s11295-011-0438-7. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

