Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2
- PMID: 24712379
- PMCID: PMC4192116
- DOI: 10.1111/adb.12139
Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2
Abstract
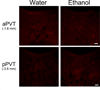
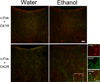
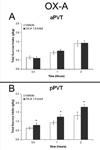
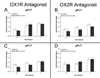
The paraventricular nucleus of the thalamus (PVT) has been shown to participate in hedonic feeding and is thought to influence drug seeking. This understudied nucleus contains anterior (aPVT) and posterior (pPVT) subregions, which receive dense projections from hypothalamic orexin/hypocretin (OX) but exhibit anatomical and functional differences. This study sought to characterize in Long-Evans rats the involvement of these PVT subregions and their OX receptor activity in consumption of the drug, ethanol. Compared with those maintained on water and chow only (water group), rats trained to drink pharmacologically relevant levels of ethanol (ethanol group) showed increased neuronal activation in the PVT, specifically the aPVT but not pPVT, as indicated by c-Fos immunoreactivity. Similar results were obtained in rats administered ethanol via oral gavage, indicating that this site-specific effect was due to ethanol exposure. In support of the involvement of OX, the ethanol group also showed increased mRNA levels of this neuropeptide in the hypothalamus and of OX 2 receptor (OX2R) but not OX 1 receptor (OX1R), again in the aPVT but not pPVT. Similarly, ethanol gavage increased double labeling of c-Fos with OX2R but not OX1R, specifically in the aPVT. Evidence directly supporting a role for aPVT OX2R in ethanol consumption was provided by results with local injections, showing ethanol intake to be enhanced by OX-A or OX-B in the aPVT but not pPVT and reduced by a local antagonist of OX2R but not OX1R. These results focus attention on the aPVT and specifically its OX2R in mediating a positive feedback relationship with ethanol intake.
Keywords: c-Fos; emotional behavior; immunohistochemistry; intermittent access; microinjection; rat.
© 2014 Society for the Study of Addiction.
Figures





References
-
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. - PubMed
-
- Brown RM, Yon-Seng Khoo S, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol. 2013:1–13. - PubMed
-
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

