T-cell selection and intestinal homeostasis
- PMID: 24712459
- PMCID: PMC4028094
- DOI: 10.1111/imr.12171
T-cell selection and intestinal homeostasis
Abstract
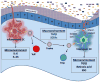
Although intestinal bacteria live deep within the body, they are topographically on the exterior surface and thus outside the host. According to the classic notion that the immune system targets non-self rather than self, these intestinal bacteria should be considered foreign and therefore attacked and eliminated. While this appears to be true for some commensal bacterial species, recent data suggest that the immune system actively becomes tolerant to many bacterial organisms. The induction or activation of regulatory T (Treg) cells that inhibit, rather than promote, inflammatory responses to commensal bacteria appears to be a central component of mucosal tolerance. Loss of this mechanism can lead to inappropriate immune reactivity toward commensal organisms, perhaps contributing to mucosal inflammation characteristic of disorders such as inflammatory bowel disease.
Keywords: T cell; T-cell receptors; inflammatory bowel disease; mucosa.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

