tTregs, pTregs, and iTregs: similarities and differences
- PMID: 24712461
- PMCID: PMC3982187
- DOI: 10.1111/imr.12160
tTregs, pTregs, and iTregs: similarities and differences
Abstract
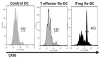
Foxp3(+) T-regulatory cells (Tregs) are primarily generated in the thymus (tTreg), but also may be generated extrathymically at peripheral sites (pTreg), or induced in cell culture (iTreg) in the presence of transforming growth factor β (TGFβ). A major unresolved issue is how these different populations of Tregs exert their suppressive function in vivo. We have developed novel systems in which the function of Tregs can be evaluated in vivo in normal mice. Our studies demonstrate that one prominent mechanism of action of polyclonal tTregs is to inhibit T-effector cell trafficking to the target organ, while antigen-specific iTregs primarily prevent T-cell priming by acting on antigen-presenting dendritic cells (DCs). Interleukin-10 (IL-10) plays an important role in the suppressive function of antigen-specific iTregs by controlling the expression of MARCH1 and CD83 on the DC. Activated tTregs may mediate infectious tolerance by delivery of cell surface-expressed TGFβ to naive responder T cells to generate pTregs. Manipulation of Treg function will require the ability to differentiate tTregs from pTregs and iTregs. The expression of the transcription factor Helios has proven to be a useful marker for the identification of stable tTregs in both mouse and human.
Keywords: Helios; IL-10; Treg cells; infectious tolerance; organ-specific autoimmunity; stability.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
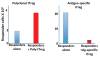


References
-
- Abbas AK, et al. Regulatory T cells: recommendations to simplify nomenclature. Nat Immunol. 2013;14:307–308. - PubMed
-
- Polansky JK, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008;38:165–1663. - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

