Total drug treatment and comorbidity in myasthenia gravis: a population-based cohort study
- PMID: 24712740
- PMCID: PMC4238850
- DOI: 10.1111/ene.12439
Total drug treatment and comorbidity in myasthenia gravis: a population-based cohort study
Abstract
Background and purpose: Comorbidity in myasthenia gravis (MG) is important for diagnosis, treatment and prognosis. Disease complexity was assessed by examining total drug treatment, immune therapy and comorbidity in a complete national MG cohort.
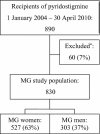
Methods: All recipients of the MG-specific drug pyridostigmine 2004-2010 registered in the compulsory Norwegian Prescription Database who met the inclusion criteria were included. The pyridostigmine group was compared with the general Norwegian population.
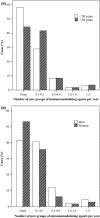
Results: Myasthenia gravis patients received co-medication more often than the controls for nearly all groups of medication, including insulins (95% confidence interval 2.0-3.7), thyroid therapy (1.7-2.5), antidepressants (1.3-1.7), anti-infectives (1.2-1.4), lipid-modifying agents (1.1-1.4) and immunomodulating agents (6.8-8.8).
Conclusions: Myasthenia gravis patients are more often treated with non-MG prescription drugs than controls, reflecting frequent co-medication and comorbidity.
Keywords: comorbidity; drug therapy; myasthenia gravis.
© 2014 The Author(s) European Journal of Neurology © 2014 EAN.
Figures
References
-
- Querol L, Illa I. Myasthenia gravis and the neuromuscular junction. Curr Opin Neurol. 2013;26:459–465. - PubMed
-
- WHO Collaborating Centre for Drug Statistics Methodology. Oslo, Norway: 2005. Norwegian Institute of Public Health Guidelines for ATC classification and DDD assignment.
-
- Skeie GO, Apostolski S, Evoli A, et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol. 2010;17:893–902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

