Genetically programmable thermoresponsive plasmonic gold/silk-elastin protein core/shell nanoparticles
- PMID: 24712906
- PMCID: PMC4002124
- DOI: 10.1021/la403559t
Genetically programmable thermoresponsive plasmonic gold/silk-elastin protein core/shell nanoparticles
Abstract
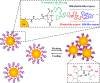
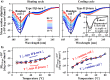
The design and development of future molecular photonic/electronic systems pose the challenge of integrating functional molecular building blocks in a controlled, tunable, and reproducible manner. The modular nature and fidelity of the biosynthesis method provides a unique chemistry approach to one-pot synthesis of environmental factor-responsive chimeric proteins capable of energy conversion between the desired forms. In this work, facile tuning of dynamic thermal response in plasmonic nanoparticles was facilitated by genetic engineering of the structure, size, and self-assembly of the shell silk-elastin-like protein polymers (SELPs). Recombinant DNA techniques were implemented to synthesize a new family of SELPs, S4E8Gs, with amino acid repeats of [(GVGVP)4(GGGVP)(GVGVP)3(GAGAGS)4] and tunable molecular weight. The temperature-reversible conformational switching between the hydrophilic random coils and the hydrophobic β-turns in the elastin blocks were programmed to between 50 and 60 °C by site-specific glycine mutation, as confirmed by variable-temperature proton NMR and circular dichroism (CD) spectroscopy, to trigger the nanoparticle aggregation. The dynamic self-aggregation/disaggregation of the Au-SELPs nanoparticles was regulated in size and pattern by the β-sheet-forming, thermally stable silk blocks, as revealed by transmission electron microscopy (TEM) and dynamic light scattering (DLS). The thermally reversible, shell dimension dependent, interparticle plasmon coupling was investigated by both variable-temperature UV-vis spectroscopy and finite-difference time-domain (FDTD)-based simulations. Good agreement between the calculated and measured spectra sheds light on design and synthesis of responsive plasmonic nanostructures by independently tuning the refractive index and size of the SELPs through genetic engineering.
Figures





Similar articles
-
Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics.Expert Opin Drug Deliv. 2015 May;12(5):779-91. doi: 10.1517/17425247.2015.989830. Epub 2014 Dec 5. Expert Opin Drug Deliv. 2015. PMID: 25476201 Free PMC article. Review.
-
Tunable self-assembly of genetically engineered silk--elastin-like protein polymers.Biomacromolecules. 2011 Nov 14;12(11):3844-50. doi: 10.1021/bm201165h. Epub 2011 Sep 30. Biomacromolecules. 2011. PMID: 21955178 Free PMC article.
-
Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery.Biomacromolecules. 2014 Mar 10;15(3):908-14. doi: 10.1021/bm4017594. Epub 2014 Feb 21. Biomacromolecules. 2014. PMID: 24527851 Free PMC article.
-
Self-Assembly of Thermoresponsive Recombinant Silk-Elastinlike Nanogels.Macromol Biosci. 2018 Jan;18(1):10.1002/mabi.201700192. doi: 10.1002/mabi.201700192. Epub 2017 Sep 4. Macromol Biosci. 2018. PMID: 28869362 Free PMC article.
-
Silk-elastinlike protein-based hydrogels for drug delivery and embolization.Adv Drug Deliv Rev. 2022 Dec;191:114579. doi: 10.1016/j.addr.2022.114579. Epub 2022 Oct 25. Adv Drug Deliv Rev. 2022. PMID: 36306893 Review.
Cited by
-
Unraveling the Molecular Mechanisms of Thermo-responsive Properties of Silk-Elastin-Like Proteins by Integrating Multiscale Modeling and Experiment.J Mater Chem B. 2018 Jun 14;6(22):3727-3734. doi: 10.1039/C8TB00819A. Epub 2018 May 3. J Mater Chem B. 2018. PMID: 30467524 Free PMC article.
-
Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics.Expert Opin Drug Deliv. 2015 May;12(5):779-91. doi: 10.1517/17425247.2015.989830. Epub 2014 Dec 5. Expert Opin Drug Deliv. 2015. PMID: 25476201 Free PMC article. Review.
-
Genetically Fusing Order-Promoting and Thermoresponsive Building Blocks to Design Hybrid Biomaterials.Chemistry. 2024 May 28;30(30):e202400582. doi: 10.1002/chem.202400582. Epub 2024 Apr 10. Chemistry. 2024. PMID: 38501912 Free PMC article. Review.
-
Synergistic Integration of Experimental and Simulation Approaches for the de Novo Design of Silk-Based Materials.Acc Chem Res. 2017 Apr 18;50(4):866-876. doi: 10.1021/acs.accounts.6b00616. Epub 2017 Feb 13. Acc Chem Res. 2017. PMID: 28191922 Free PMC article. Review.
-
Design of Multistimuli Responsive Hydrogels Using Integrated Modeling and Genetically Engineered Silk-Elastin-Like Proteins.Adv Funct Mater. 2016 Jun 20;26(23):4113-4123. doi: 10.1002/adfm.201600236. Epub 2016 Apr 15. Adv Funct Mater. 2016. PMID: 28670244 Free PMC article.
References
-
- Fan J. A.; Wu C.; Bao K.; Bao J.; Bardhan R.; Halas N. J.; Manoharan V. N.; Nordlander P.; Shvets G.; Capasso F. Self-assembled plasmonic nanoparticle clusters. Science 2010, 328, 1135–1138. - PubMed
-
- Chen Y. S.; Hong M. Y.; Huang G. S. A protein transistor made of an antibody molecule and two gold nanoparticles. Nat. Nanotechnol. 2012, 7, 197–203. - PubMed
-
- Gao B.; Arya G.; Tao A. R. Self-orienting nanocubes for the assembly of plasmonic nanojunctions. Nat. Nanotechnol. 2012, 7, 433–437. - PubMed
-
- Shafiei F.; Wu C.; Wu Y.; Khanikaev A. B.; Putzke P.; Singh A.; Li X.; Shvets S. Plasmonic nano-protractor based on polarization spectro-tomography. Nat. Photonics 2013, 7, 367–372.
-
- Cobley C. M.; Chen J. Y.; Cho E. C.; Wang L. V.; Xia Y. N. Gold nanostructures: a class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

