Mind the gaps--the epidemiology of poor-quality anti-malarials in the malarious world--analysis of the WorldWide Antimalarial Resistance Network database
- PMID: 24712972
- PMCID: PMC4021408
- DOI: 10.1186/1475-2875-13-139
Mind the gaps--the epidemiology of poor-quality anti-malarials in the malarious world--analysis of the WorldWide Antimalarial Resistance Network database
Abstract
Background: Poor quality medicines threaten the lives of millions of patients and are alarmingly common in many parts of the world. Nevertheless, the global extent of the problem remains unknown. Accurate estimates of the epidemiology of poor quality medicines are sparse and are influenced by sampling methodology and diverse chemical analysis techniques. In order to understand the existing data, the Antimalarial Quality Scientific Group at WWARN built a comprehensive, open-access, global database and linked Antimalarial Quality Surveyor, an online visualization tool. Analysis of the database is described here, the limitations of the studies and data reported, and their public health implications discussed.
Methods: The database collates customized summaries of 251 published anti-malarial quality reports in English, French and Spanish by time and location since 1946. It also includes information on assays to determine quality, sampling and medicine regulation.
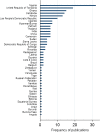
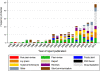
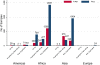
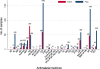
Results: No publicly available reports for 60.6% (63) of the 104 malaria-endemic countries were found. Out of 9,348 anti-malarials sampled, 30.1% (2,813) failed chemical/packaging quality tests with 39.3% classified as falsified, 2.3% as substandard and 58.3% as poor quality without evidence available to categorize them as either substandard or falsified. Only 32.3% of the reports explicitly described their definitions of medicine quality and just 9.1% (855) of the samples collected in 4.6% (six) surveys were conducted using random sampling techniques. Packaging analysis was only described in 21.5% of publications and up to twenty wrong active ingredients were found in falsified anti-malarials.
Conclusions: There are severe neglected problems with anti-malarial quality but there are important caveats to accurately estimate the prevalence and distribution of poor quality anti-malarials. The lack of reports in many malaria-endemic areas, inadequate sampling techniques and inadequate chemical analytical methods and instrumental procedures emphasizes the need to interpret medicine quality results with caution. The available evidence demonstrates the need for more investment to improve both sampling and analytical methodology and to achieve consensus in defining different types of poor quality medicines.
Figures

Comment in
-
Poor-quality medicines: from knowledge to control and prevention.Pathog Glob Health. 2014 Jun;108(4):171-2. doi: 10.1179/2047772414Z.000000000207. Pathog Glob Health. 2014. PMID: 24954880 Free PMC article. No abstract available.
References
-
- World Health Organization. World Malaria Report 2012. 2012. [cited 2013 March, 17th]; Available from: http://www.who.int/malaria/publications/world_malaria_report_2012/en/
-
- United Nations Human Rights Council. Access to medicines in the context of the right of everyone to the to the enjoyment of the highest attainable standard of physical and mental health. Twenty-third session, Agenda item 3, Promotion and protection of all human rights, civil, political, economic, social and cultural rights, including the right to development 2013 [cited 2013 June 20th]; Available from: http://ap.ohchr.org/documents/dpage_e.aspx?si=A/HRC/23/L.10/rev.1.
-
- Caudron JM, Ford N, Henkens M, Macé C, Kiddle-Monroe R, Pinel J. Substandard medicines in resource-poor settings: a problem that can no longer be ignored. Médicaments sous-standard dans les milieux défavorisés: un problème qui ne peut plus être ignoré. Revue Medicamentos sub-estándar en lugares con pocos recursos: un problema que no puede ser ignorado por más tiempo. Revisión. Tropical Med Int Health. 2008;13(8):1062–1072. doi: 10.1111/j.1365-3156.2008.02106.x. - DOI - PubMed
-
- Institute of Medicine of the National Academies. Countering the Problem of Falsified and Substandard Drugs. National Academy of Sciences. The National Academies Press; 2013. Available from: http://www.iom.edu/reports/2013/countering-the-problem-of-falsified-and-.... - PubMed
-
- Newton PN, Green MD, Mildenhall DC, Plancon A, Nettey H, Nyadong L, Hostetler DM, Swamidoss I, Harris GA, Powell K, Timmermans AE, Amin AA, Opuni SK, Barbereau S, Faurant C, Soong RCW, Faure K, Thevanayagam J, Fernandes P, Kaur H, Angus B, Stepniewska K, Guerin PJ, Fernandez FM. Poor quality vital antimalarials in Africa – an urgent neglected public health priority. Malar J. 2011;10:352. doi: 10.1186/1475-2875-10-352. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

