BDNF contributes to both rapid and homeostatic alterations in AMPA receptor surface expression in nucleus accumbens medium spiny neurons
- PMID: 24712995
- PMCID: PMC4410784
- DOI: 10.1111/ejn.12422
BDNF contributes to both rapid and homeostatic alterations in AMPA receptor surface expression in nucleus accumbens medium spiny neurons
Abstract
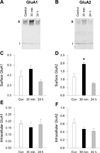
Brain-derived neurotrophic factor (BDNF) plays a critical role in plasticity at glutamate synapses and in the effects of repeated cocaine exposure. We recently showed that intracranial injection of BDNF into the rat nucleus accumbens (NAc), a key region for cocaine addiction, rapidly increases α-amino-3-hyroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR) surface expression. To further characterize BDNF's role in both rapid AMPAR trafficking and slower, homeostatic changes in AMPAR surface expression, we investigated the effects of acute (30 min) and long-term (24 h) treatment with BDNF on AMPAR distribution in NAc medium spiny neurons from postnatal rats co-cultured with mouse prefrontal cortex neurons to restore excitatory inputs. Immunocytochemical studies showed that acute BDNF treatment increased cell surface GluA1 and GluA2 levels, as well as their co-localization, on NAc neurons. This effect of BDNF, confirmed using a protein crosslinking assay, was dependent on ERK but not AKT signaling. In contrast, long-term BDNF treatment decreased AMPAR surface expression on NAc neurons. Based on this latter result, we tested the hypothesis that BDNF plays a role in AMPAR 'scaling down' in response to a prolonged increase in neuronal activity produced by bicuculline (24 h). Supporting this hypothesis, decreasing BDNF signaling with the extracellular BDNF scavenger TrkB-Fc prevented the scaling down of GluA1 and GluA2 surface levels in NAc neurons normally produced by bicuculline. In conclusion, BDNF exerts bidirectional effects on NAc AMPAR surface expression, depending on duration of exposure. Furthermore, BDNF's involvement in synaptic scaling in the NAc differs from its previously described role in the visual cortex.
Keywords: co-culture; mouse; rat; receptor trafficking; synaptic scaling.
© 2014 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. - PubMed
-
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsey RM, Wiegland SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. - PubMed
-
- Bahi A, Boyer F, Dreyer J-L. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned place preference and reinstatement in rats. Psychopharmacology. 2008;199:169–182. - PubMed
-
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur. J. Neurosci. 2007;26:757–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

