Mycobacterial RNA polymerase requires a U-tract at intrinsic terminators and is aided by NusG at suboptimal terminators
- PMID: 24713321
- PMCID: PMC3993855
- DOI: 10.1128/mBio.00931-14
Mycobacterial RNA polymerase requires a U-tract at intrinsic terminators and is aided by NusG at suboptimal terminators
Abstract
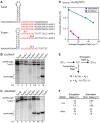
Intrinsic terminators, which encode GC-rich RNA hairpins followed immediately by a 7-to-9-nucleotide (nt) U-rich "U-tract," play principal roles of punctuating and regulating transcription in most bacteria. However, canonical intrinsic terminators with strong U-tracts are underrepresented in some bacterial lineages, notably mycobacteria, leading to proposals that their RNA polymerases stop at noncanonical intrinsic terminators encoding various RNA structures lacking U-tracts. We generated recombinant forms of mycobacterial RNA polymerase and its major elongation factors NusA and NusG to characterize mycobacterial intrinsic termination. Using in vitro transcription assays devoid of possible mycobacterial contaminants, we established that mycobacterial RNA polymerase terminates more efficiently than Escherichia coli RNA polymerase at canonical terminators with imperfect U-tracts but does not terminate at putative terminators lacking U-tracts even in the presence of mycobacterial NusA and NusG. However, mycobacterial NusG exhibits a novel termination-stimulating activity that may allow intrinsic terminators with suboptimal U-tracts to function efficiently. IMPORTANCE Bacteria rely on transcription termination to define and regulate units of gene expression. In most bacteria, precise termination and much regulation by attenuation are accomplished by intrinsic terminators that encode GC-rich hairpins and U-tracts necessary to disrupt stable transcription elongation complexes. Thus, the apparent dearth of canonical intrinsic terminators with recognizable U-tracts in mycobacteria is of significant interest both because noncanonical intrinsic terminators could reveal novel routes to destabilize transcription complexes and because accurate understanding of termination is crucial for strategies to combat mycobacterial diseases and for computational bioinformatics generally. Our finding that mycobacterial RNA polymerase requires U-tracts for intrinsic termination, which can be aided by NusG, will guide future study of mycobacterial transcription and aid improvement of predictive algorithms to annotate bacterial genome sequences.
Figures



References
-
- Richardson J, Greenblatt J. 1996. Control of RNA chain elongation and termination, p 822–848 In Neidhardt F, Curtiss R, III, Ingraham J, Lin E, Low K, Magasanik B, Reznikoff W, Riley M, Schaechter M, Umbarger H. (ed), Escherichia coli and Salmonella: cellular and molecular biology, vol 1 ASM Press, Washington, DC
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
