Hepatic nuclear corepressor 1 regulates cholesterol absorption through a TRβ1-governed pathway
- PMID: 24713658
- PMCID: PMC4001554
- DOI: 10.1172/JCI73419
Hepatic nuclear corepressor 1 regulates cholesterol absorption through a TRβ1-governed pathway
Abstract
Transcriptional coregulators are important components of nuclear receptor (NR) signaling machinery and provide additional mechanisms for modulation of NR activity. Expression of a mutated nuclear corepressor 1 (NCoR1) that lacks 2 NR interacting domains (NCoRΔID) in the liver leads to elevated expression of genes regulated by thyroid hormone receptor (TR) and liver X receptor (LXR), both of which control hepatic cholesterol metabolism. Here, we demonstrate that expression of NCoRΔID in mouse liver improves dietary cholesterol tolerance in an LXRα-independent manner. NCoRΔID-associated cholesterol tolerance was primarily due to diminished intestinal cholesterol absorption as the result of changes in the composition and hydrophobicity of the bile salt pool. Alterations of the bile salt pool were mediated by increased expression of genes encoding the bile acid metabolism enzymes CYP27A1 and CYP3A11 as well as canalicular bile salt pump ABCB11. We have determined that these genes are regulated by thyroid hormone and that TRβ1 is recruited to their regulatory regions. Together, these data indicate that interactions between NCoR1 and TR control a specific pathway involved in regulation of cholesterol metabolism and clearance.
Figures
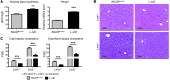
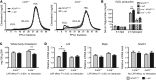
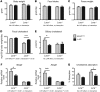
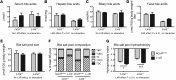

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK056626/DK/NIDDK NIH HHS/United States
- R56 DK056123/DK/NIDDK NIH HHS/United States
- R29 DK048873/DK/NIDDK NIH HHS/United States
- R01 DK056123/DK/NIDDK NIH HHS/United States
- R01 DK048873/DK/NIDDK NIH HHS/United States
- DK048873/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- R01 DK078090/DK/NIDDK NIH HHS/United States
- R37 DK048873/DK/NIDDK NIH HHS/United States
- DK078090/DK/NIDDK NIH HHS/United States
- DK056123/DK/NIDDK NIH HHS/United States
- T32 DK007516/DK/NIDDK NIH HHS/United States
- DK056626/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

