Comparison of two doses of elemental iron in the treatment of latent iron deficiency: efficacy, side effects and blinding capabilities
- PMID: 24714351
- PMCID: PMC4011041
- DOI: 10.3390/nu6041394
Comparison of two doses of elemental iron in the treatment of latent iron deficiency: efficacy, side effects and blinding capabilities
Abstract
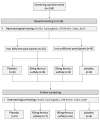
Adherence to iron supplementation can be compromised due to side effects, and these limit blinding in studies of iron deficiency. No studies have reported an efficacious iron dose that allows participants to remain blinded. This pilot study aimed to determine a ferrous sulfate dose that improves iron stores, while minimising side effects and enabling blinding. A double-blinded RCT was conducted in 32 women (18-35 years): 24 with latent iron deficiency (serum ferritin < 20 µg/L) and 8 iron sufficient controls. Participants with latent iron deficiency were randomised to 60 mg or 80 mg elemental iron or to placebo, for 16 weeks. The iron sufficient control group took placebo. Treatment groups (60 mg n = 7 and 80 mg n = 6) had significantly higher ferritin change scores than placebo groups (iron deficient n = 5 and iron sufficient n = 6), F(1, 23) = 8.46, p ≤ 0.01. Of the 24 who completed the trial, 10 participants (77%) on iron reported side effects, compared with 5 (45%) on placebo, but there were no differences in side effects (p = 0.29), or compliance (p = 0.60) between iron groups. Nine (69%) participants on iron, and 11 (56%) on placebo correctly guessed their treatment allocation. Both iron doses were equally effective in normalising ferritin levels. Although reported side-effects were similar for both groups, a majority of participants correctly guessed their treatment group.
Figures
References
-
- World Health Organization Iron deficiency anaemia: Assessment, prevention, and control. A guide for programme managers. [(accessed on 10 August 2013)]. Available online: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_c....
-
- Fayet F., Flood V., Petocz P., Samman S. Relative and biomarker-based validity of a food frequency questionnaire that measures the intakes of vitamin B(12), folate, iron, and zinc in young women. Nutr. Res. 2010;31:14–20. - PubMed
-
- Mora J.O. Iron supplementation: Overcoming technical and practical barriers. J. Nutr. 2002;132:S853–S855. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical