Comprehensive evaluation of peripheral nerve regeneration in the acute healing phase using tissue clearing and optical microscopy in a rodent model
- PMID: 24714405
- PMCID: PMC3979924
- DOI: 10.1371/journal.pone.0094054
Comprehensive evaluation of peripheral nerve regeneration in the acute healing phase using tissue clearing and optical microscopy in a rodent model
Abstract
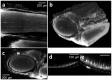
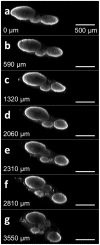
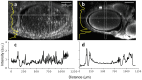
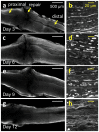
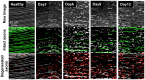
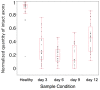
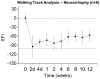
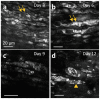
Peripheral nerve injury (PNI), a common injury in both the civilian and military arenas, is usually associated with high healthcare costs and with patients enduring slow recovery times, diminished quality of life, and potential long-term disability. Patients with PNI typically undergo complex interventions but the factors that govern optimal response are not fully characterized. A fundamental understanding of the cellular and tissue-level events in the immediate postoperative period is essential for improving treatment and optimizing repair. Here, we demonstrate a comprehensive imaging approach to evaluate peripheral nerve axonal regeneration in a rodent PNI model using a tissue clearing method to improve depth penetration while preserving neural architecture. Sciatic nerve transaction and end-to-end repair were performed in both wild type and thy-1 GFP rats. The nerves were harvested at time points after repair before undergoing whole mount immunofluorescence staining and tissue clearing. By increasing the optic depth penetration, tissue clearing allowed the visualization and evaluation of Wallerian degeneration and nerve regrowth throughout entire sciatic nerves with subcellular resolution. The tissue clearing protocol did not affect immunofluorescence labeling and no observable decrease in the fluorescence signal was observed. Large-area, high-resolution tissue volumes could be quantified to provide structural and connectivity information not available from current gold-standard approaches for evaluating axonal regeneration following PNI. The results are suggestive of observed behavioral recovery in vivo after neurorrhaphy, providing a method of evaluating axonal regeneration following repair that can serve as an adjunct to current standard outcomes measurements. This study demonstrates that tissue clearing following whole mount immunofluorescence staining enables the complete visualization and quantitative evaluation of axons throughout nerves in a PNI model. The methods developed in this study could advance PNI research allowing both researchers and clinicians to further understand the individual events of axonal degeneration and regeneration on a multifaceted level.
Conflict of interest statement
Figures

References
-
- Isaacs J (2010) Treatment of acute peripheral nerve injuries: current concepts. The Journal of hand surgery 35: 491–497. - PubMed
-
- Wiberg M, Terenghi G (2003) Will it be possible to produce peripheral nerves? Surgical technology international 11: 303. - PubMed
-
- Birch R, Eardley WGP, Ramasamy A, Brown K, Shenoy R, et al. (2012) Nerve injuries sustained during warfare PART I - EPIDEMIOLOGY. Journal of Bone and Joint Surgery-British Volume 94B: 523–528. - PubMed
-
- Maricevic A, Erceg M (1997) War injuries to the extremities. Military medicine 162: 808–811. - PubMed
-
- Campbell WW (2008) Evaluation and management of peripheral nerve injury. Clinical neurophysiology 119: 1951–1965. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

