A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy
- PMID: 24714414
- PMCID: PMC4106642
- DOI: 10.1093/ndt/gfu069
A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy
Abstract
Background: H.P. Acthar(®) Gel is currently the only Food and Drug Administration therapy approved for the treatment of nephrotic syndrome. Active drug ingredients include structurally related melanocortin peptides that bind to cell surface G-protein-coupled receptors known as melanocortin receptors, which are expressed in glomerular podocytes. In animal models of membranous nephropathy, stimulation has been demonstrated to reduce podocyte injury and loss. We hypothesized that H.P. Acthar(®) Gel would improve symptoms of the nephrotic syndrome in patients with idiopathic membranous nephropathy.
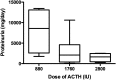
Methods: Twenty patients received a subcutaneous dose of 40 or 80 IU twice weekly. Changes in proteinuria, albumin, cholesterol profile, estimated glomerular filtration rate and serum anti-PLA2R antibodies were assessed at baseline and in response to treatment along with tolerance and safety.
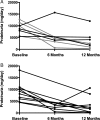
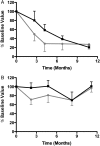
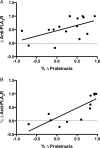
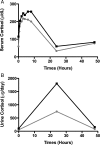
Results: Baseline characteristics included mean proteinuria (9.1 ± 3.4 g/day), albumin (2.7 ± 0.8 g/dL), estimated glomerular filtration rate (77 ± 30 mL/min) along with elevated total and low-density lipoprotein (LDL) cholesterol. By 12 months of follow-up, there was a significant improvement in proteinuria in the entire cohort, decreasing to 3.87 ± 4.24 g/day (P < 0.001) with significant improvements in serum albumin, total and LDL cholesterol. A >50% decrease in proteinuria was noted in 65% of the patients with a trend toward better outcomes among patients who received greater cumulative doses. No significant adverse effects were documented. Clearing of serum anti-PLA2R antibodies prior to or in parallel with proteinuria improvement was noted in some, but not all patients.
Conclusions: H.P. Acthar(®) Gel is a potential therapy for nephrotic syndrome secondary to idiopathic membranous nephropathy that deserves further study.
Keywords: ACTH (H.P. Acthar® Gel); membranous nephropathy; nephrotic syndrome.
© The Author 2014. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
References
-
- Swaminathan S, Leung N, Lager DJ, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1:483–487. - PubMed
-
- MacTier R, Boulton Jones JM, Payton CD, et al. The natural history of membranous nephropathy in the West of Scotland. Q J Med. 1986;60:793–802. - PubMed
-
- Noel LH, Zanetti M, Droz D, et al. Long-term prognosis of idiopathic membranous glomerulonephritis. Study of 116 untreated patients. Am J Med. 1979;66:82–90. - PubMed
-
- Zucchelli P, Ponticelli C, Cagnoli L, et al. Long-term outcome of idiopathic membranous nephropathy with nephrotic syndrome. Nephrol Dial Transplant. 1987;2:73–78. - PubMed
-
- Davison AM, Cameron JS, Kerr DN, et al. The natural history of renal function in untreated idiopathic membranous glomerulonephritis in adults. Clin Nephrol. 1984;22:61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

