Influenza vaccine immunogenicity in patients with primary central nervous system malignancy
- PMID: 24714522
- PMCID: PMC4232079
- DOI: 10.1093/neuonc/nou051
Influenza vaccine immunogenicity in patients with primary central nervous system malignancy
Abstract
Background: Patients with central nervous system (CNS) malignancies represent an "at-risk" population for contracting influenza, particularly if they are receiving ongoing chemotherapy, radiation, and/or glucocorticoid treatment. The Centers for Disease Control endorses vaccination for these patients, although data are not available to indicate whether they mount an immunologic response adequate to achieve clinical protection.
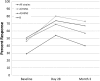
Methods: A pilot prospective cohort study was designed to evaluate the immunogenicity of the standard-dose trivalent inactivated influenza vaccine in patients with malignant CNS tumors. Baseline data collection included diagnosis, chemotherapy, timing of chemotherapy or radiation relative to vaccination, and glucocorticoid dose. Serum samples were collected at baseline, day 14, day 28, and month 3 following vaccination. Samples were tested using hemagglutinin inhibition to determine seroconversion (4-fold rise in titer) and seroprotection (titer >1:40).
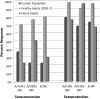
Results: A total of 38 patients were enrolled (mean age, 54 years ±13.5 years, 60.5% male, 94.7% Caucasian, and 5.3% African American). CNS tumor diagnoses included glioblastoma multiforme (55.2%), other high-grade glioma (13.2%), low-grade glioma (15.8%), and primary CNS lymphoma (15.8%). At enrollment, 20 patients (52.6%) were taking glucocorticoids, 25 (65.8%) were on active chemotherapy, and 3 (7.9%) were undergoing radiation. Seroconversion rates at day 28 for the A/H1N1, A/H3N2, and B strains were 37%, 23% and 23%, respectively. Seroprotection was 80%, 69%, and 74%, respectively. All rates were significantly lower than published rates in healthy adults (P < .001).
Conclusion: Influenza vaccine immunogenicity is significantly reduced in patients with CNS malignancies. Future studies are needed to determine the causative etiologies and appropriate vaccination strategies.
Keywords: central nervous system malignancy; chemotherapy; influenza vaccine.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Influenza vaccine immunogenicity in 6- to 23-month-old children: are identical antigens necessary for priming?Pediatrics. 2006 Sep;118(3):e570-8. doi: 10.1542/peds.2006-0198. Pediatrics. 2006. PMID: 16950948 Clinical Trial.
-
Immunization with trivalent inactivated influenza vaccine in partially immunized toddlers.Pediatrics. 2006 Sep;118(3):e579-85. doi: 10.1542/peds.2006-0201. Pediatrics. 2006. PMID: 16950949 Clinical Trial.
-
Immunogenicity of trivalent influenza vaccine in patients with lung cancer undergoing anticancer chemotherapy.Hum Vaccin Immunother. 2017 Mar 4;13(3):543-550. doi: 10.1080/21645515.2016.1246094. Epub 2016 Nov 7. Hum Vaccin Immunother. 2017. PMID: 27820665 Free PMC article.
-
The safety and immunogenicity of trivalent inactivated influenza vaccination: a study of maternal-cord blood pairs in Taiwan.PLoS One. 2013 Jun 6;8(6):e62983. doi: 10.1371/journal.pone.0062983. Print 2013. PLoS One. 2013. PMID: 23762229 Free PMC article. Clinical Trial.
-
Immunogenicity and Safety of Reduced-Dose Intradermal vs Intramuscular Influenza Vaccines: A Systematic Review and Meta-analysis.JAMA Netw Open. 2021 Feb 1;4(2):e2035693. doi: 10.1001/jamanetworkopen.2020.35693. JAMA Netw Open. 2021. PMID: 33560425 Free PMC article.
Cited by
-
Copper homeostasis and cuproptosis in central nervous system diseases.Cell Death Dis. 2024 Nov 21;15(11):850. doi: 10.1038/s41419-024-07206-3. Cell Death Dis. 2024. PMID: 39567497 Free PMC article. Review.
-
Immunogenicity of high-dose influenza vaccination in patients with primary central nervous system malignancy.Neurooncol Pract. 2018 Aug;5(3):176-183. doi: 10.1093/nop/npx035. Epub 2018 Jan 6. Neurooncol Pract. 2018. PMID: 31385974 Free PMC article.
-
Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications.Front Oncol. 2021 Oct 28;11:770561. doi: 10.3389/fonc.2021.770561. eCollection 2021. Front Oncol. 2021. PMID: 34778089 Free PMC article. Review.
-
Vaccination of people with solid tumors and diabetes: existing evidence and recommendations. A position statement from a multidisciplinary panel of scientific societies.J Endocrinol Invest. 2025 Aug;48(8):1717-1738. doi: 10.1007/s40618-025-02586-5. Epub 2025 Apr 23. J Endocrinol Invest. 2025. PMID: 40266540 Free PMC article.
References
-
- Kempe A, Hall CB, MacDonald NE, et al. Infuenza in children with cancer. J Pediatr. 1989;115(1):33–39. - PubMed
-
- Baerk WH, Mulloly JP. Pneumonia and influenza deaths during epidemics: Implications for prevention. Arch Intern Med. 1982;142(1):85–89. - PubMed
-
- Cooksley CD, Avritscher EB, Bekele BN, et al. Epidemiology and outcomes of serious influenza-related infections in cancer population. Cancer. 2005;104(3):618–628. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. - PubMed
-
- Whimbley E, Elting LS, Cough RB, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13(4):437–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

