Translational control by heme-regulated eIF2α kinase during erythropoiesis
- PMID: 24714526
- PMCID: PMC4124034
- DOI: 10.1097/MOH.0000000000000030
Translational control by heme-regulated eIF2α kinase during erythropoiesis
Abstract
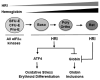
Purpose of review: This review will provide an overview of the translational regulation of globin mRNAs and integrated stress response (ISR) during erythropoiesis by heme-regulated eIF2α kinase (HRI). HRI is an intracellular heme sensor that coordinates heme and globin synthesis in erythropoiesis by inhibiting protein synthesis of globins and heme biosynthetic enzymes during heme deficiency.
Recent findings: It has been demonstrated recently that HRI also activates the eIF2αP-activating transcription factor 4 (ATF4) ISR in primary erythroid precursors to combat oxidative stress. During chronic iron/heme deficiency in vivo, this HRI-eIF2αP-ATF4 signaling is necessary both to reduce oxidative stress and to promote erythroid differentiation. Augmenting eIF2αP signaling by the small molecule salubrinal, which inhibits dephosphorylation of eIF2αP, reduces excess α-globin synthesis and enhances translation of ATF4 mRNA in mouse β-thalassemic erythroid precursors. Intriguingly, salubrinal treatment of differentiating human CD34⁺ cells in culture increases fetal hemoglobin production with a concomitant decrease of adult hemoglobin by a posttranscriptional mechanism.
Summary: HRI-eIF2αP-ATF4 stress signaling is important not only to inhibit excess globin synthesis during erythropoiesis, but is also critical for adaptation to oxidative stress and for enhancing effective erythropoiesis. Modulation of this signaling pathway with small chemicals may provide a novel therapy for hemoglobinopathy.
Conflict of interest statement
There is no conflict of interests.
Figures



References
-
- Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337–42. Epub 2011/05/20. - PubMed
-
- Kingsley PD, Greenfest-Allen E, Frame JM, Bushnell TP, Malik J, McGrath KE, et al. Ontogeny of erythroid gene expression. Blood. 2013;121(6):e5–e13. Epub 2012/12/18 This is the first paper examining the global gene expression of distinct cell population during both primitive and definirtive erythropoiesis. - PMC - PubMed
-
- Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89(1):1–25. - PubMed
-
- Taketani S. Aquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku J Exp Med. 2005;205(4):297–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

