Is HCV core antigen a reliable marker of viral load? An evaluation of HCV core antigen automated immunoassay
- PMID: 24714621
- PMCID: PMC3959936
Is HCV core antigen a reliable marker of viral load? An evaluation of HCV core antigen automated immunoassay
Abstract
Background: Hepatitis C viral (HCV) load detection and quantification is routinely accomplished by HCV RNA measurement, an expensive but essential test, both for the diagnosis and treatment of chronic hepatitis C (CHC). HCV core antigen (Ag) testing has been suggested as an attractive alternative to molecular diagnostics. The aim of the study was to evaluate an automated chemiluminescent immunoassay (CLIA) for HCV core Ag measurement in comparison to quantitative HCV RNA determination.
Methods: HCV Ag was measured in 105 anti-HCV positive patients, from which 89 were HCV RNA positive with CHC and 16 HCV RNA negative after spontaneous HCV clearance. Viral load was quantified with branched DNA (bDNA, Versant, Siemens). Sera were stored at -70°C and then tested with the Architect HCV Ag test (Abbott Laboratories), a two-step CLIA assay, with high throughput and minimal handling of the specimens. Statistical analysis was performed on logarithmically transformed values.
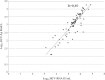
Results: HCV-Ag was detectable and quantifiable in 83/89 and in grey zone in 4/89 HCV RNA positive sera. HCV-Ag was undetectable in all 16 HCV RNA negative samples. The sample with the lowest viral load that tested positive for HCV-Ag contained 1200 IU/mL HCV RNA. There was a positive correlation between HCV RNA and HCV-Ag (r=0.89). The HCV RNA/ HCV Ag ratio varied from 1.5 to 3.25.
Conclusion: The HCV core Ag is an easy test with comparable sensitivity (>90%) and satisfactory correlation with the HCV RNA bDNA assay. Its role in diagnostics and other clinical applications has to be determined based on cost effectiveness.
Keywords: Core antigen; HCV RNA; hepatitis C.
Conflict of interest statement
Conflict of Interest: None
Figures
Similar articles
-
Comparative evaluation of the total hepatitis C virus core antigen, branched-DNA, and amplicor monitor assays in determining viremia for patients with chronic hepatitis C during interferon plus ribavirin combination therapy.J Clin Microbiol. 2003 Jul;41(7):3212-20. doi: 10.1128/JCM.41.7.3212-3220.2003. J Clin Microbiol. 2003. PMID: 12843066 Free PMC article.
-
Hepatitis C virus core antigen assay: an alternative method for hepatitis C diagnosis.Ann Clin Biochem. 2017 Mar;54(2):279-285. doi: 10.1177/0004563216661218. Epub 2016 Oct 13. Ann Clin Biochem. 2017. PMID: 27614354
-
Hepatitis C virus core antigen: analytical performances, correlation with viremia and potential applications of a quantitative, automated immunoassay.J Clin Virol. 2011 Aug;51(4):264-9. doi: 10.1016/j.jcv.2011.05.003. Epub 2011 May 28. J Clin Virol. 2011. PMID: 21621454
-
Current position of viral load versus hepatitis C core antigen testing.Enferm Infecc Microbiol Clin (Engl Ed). 2020 Jan;38 Suppl 1:12-18. doi: 10.1016/j.eimc.2020.02.003. Enferm Infecc Microbiol Clin (Engl Ed). 2020. PMID: 32111360 Review. English, Spanish.
-
Can Hepatitis C Virus Antigen Testing Replace Ribonucleic Acid Polymearse Chain Reaction Analysis for Detecting Hepatitis C Virus? A Systematic Review.Open Forum Infect Dis. 2017 May 26;4(2):ofw252. doi: 10.1093/ofid/ofw252. eCollection 2017 Spring. Open Forum Infect Dis. 2017. PMID: 28567430 Free PMC article. Review.
Cited by
-
Hepatitis C core antigen testing to diagnose active hepatitis C infection among haemodialysis patients.BMC Nephrol. 2020 Nov 13;21(1):480. doi: 10.1186/s12882-020-02154-4. BMC Nephrol. 2020. PMID: 33187498 Free PMC article.
-
Aptamer-based biosensors for virus protein detection.Trends Analyt Chem. 2022 Dec;157:116738. doi: 10.1016/j.trac.2022.116738. Epub 2022 Jul 19. Trends Analyt Chem. 2022. PMID: 35874498 Free PMC article. Review.
-
Detection of Hepatitis C Virus Core Protein in Serum Using Aptamer-Functionalized AFM Chips.Micromachines (Basel). 2019 Feb 15;10(2):129. doi: 10.3390/mi10020129. Micromachines (Basel). 2019. PMID: 30781415 Free PMC article.
-
Hepatitis C Virus-Core Antigen: Implications in Diagnostic, Treatment Monitoring and Clinical Outcomes.Viruses. 2024 Nov 29;16(12):1863. doi: 10.3390/v16121863. Viruses. 2024. PMID: 39772172 Free PMC article. Review.
-
Hepatitis C Core Antigen Testing for Diagnosis of Hepatitis C Virus Infection: A Systematic Review and Meta-analysis.Ann Intern Med. 2016 Sep 6;165(5):345-55. doi: 10.7326/M16-0065. Epub 2016 Jun 21. Ann Intern Med. 2016. PMID: 27322622 Free PMC article.
References
-
- Chevaliez S. Virological tools to diagnose and monitor hepatitis C virus infection. Clin Microbiol Infect. 2011;17:116–121. - PubMed
-
- Bouvier Alias M, Patel K, Dahari H, et al. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology. 2002;36:211–218. - PubMed
-
- Morota K, Fujinami R, Kinukawa H, et al. A new sensitive and automated chemiluminescent microparticle immunoassay for quantitative determination of hepatitis C virus core antigen. J Virol Methods. 2009;157:8–14. - PubMed
-
- Lee SC, Antony A, Lee N, et al. Improved version 2.0 qualitative and quantitative AMPLICOR reverse transcription-PCR tests for hepatitis C virus RNA: calibration to international units, enhanced genotype reactivity, and performance characteristics. J Clin Microbiol. 2000;38:4171–4179. - PMC - PubMed
-
- Alter HJ, Sanchez-Pescador R, Urdea MS, et al. Evaluation of branched DNA signal amplification for the detection of hepatitis C virus RNA. J Viral Hepat. 1995;2:121–132. - PubMed
LinkOut - more resources
Full Text Sources