Is HCV core antigen a reliable marker of viral load? An evaluation of HCV core antigen automated immunoassay
- PMID: 24714621
- PMCID: PMC3959936
Is HCV core antigen a reliable marker of viral load? An evaluation of HCV core antigen automated immunoassay
Abstract
Background: Hepatitis C viral (HCV) load detection and quantification is routinely accomplished by HCV RNA measurement, an expensive but essential test, both for the diagnosis and treatment of chronic hepatitis C (CHC). HCV core antigen (Ag) testing has been suggested as an attractive alternative to molecular diagnostics. The aim of the study was to evaluate an automated chemiluminescent immunoassay (CLIA) for HCV core Ag measurement in comparison to quantitative HCV RNA determination.
Methods: HCV Ag was measured in 105 anti-HCV positive patients, from which 89 were HCV RNA positive with CHC and 16 HCV RNA negative after spontaneous HCV clearance. Viral load was quantified with branched DNA (bDNA, Versant, Siemens). Sera were stored at -70°C and then tested with the Architect HCV Ag test (Abbott Laboratories), a two-step CLIA assay, with high throughput and minimal handling of the specimens. Statistical analysis was performed on logarithmically transformed values.
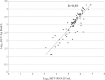
Results: HCV-Ag was detectable and quantifiable in 83/89 and in grey zone in 4/89 HCV RNA positive sera. HCV-Ag was undetectable in all 16 HCV RNA negative samples. The sample with the lowest viral load that tested positive for HCV-Ag contained 1200 IU/mL HCV RNA. There was a positive correlation between HCV RNA and HCV-Ag (r=0.89). The HCV RNA/ HCV Ag ratio varied from 1.5 to 3.25.
Conclusion: The HCV core Ag is an easy test with comparable sensitivity (>90%) and satisfactory correlation with the HCV RNA bDNA assay. Its role in diagnostics and other clinical applications has to be determined based on cost effectiveness.
Keywords: Core antigen; HCV RNA; hepatitis C.
Conflict of interest statement
Conflict of Interest: None
Figures
References
-
- Chevaliez S. Virological tools to diagnose and monitor hepatitis C virus infection. Clin Microbiol Infect. 2011;17:116–121. - PubMed
-
- Bouvier Alias M, Patel K, Dahari H, et al. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology. 2002;36:211–218. - PubMed
-
- Morota K, Fujinami R, Kinukawa H, et al. A new sensitive and automated chemiluminescent microparticle immunoassay for quantitative determination of hepatitis C virus core antigen. J Virol Methods. 2009;157:8–14. - PubMed
-
- Lee SC, Antony A, Lee N, et al. Improved version 2.0 qualitative and quantitative AMPLICOR reverse transcription-PCR tests for hepatitis C virus RNA: calibration to international units, enhanced genotype reactivity, and performance characteristics. J Clin Microbiol. 2000;38:4171–4179. - PMC - PubMed
-
- Alter HJ, Sanchez-Pescador R, Urdea MS, et al. Evaluation of branched DNA signal amplification for the detection of hepatitis C virus RNA. J Viral Hepat. 1995;2:121–132. - PubMed
LinkOut - more resources
Full Text Sources