Podosome organization drives osteoclast-mediated bone resorption
- PMID: 24714644
- PMCID: PMC4198343
- DOI: 10.4161/cam.27840
Podosome organization drives osteoclast-mediated bone resorption
Abstract
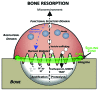
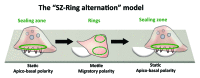
Osteoclasts are the cells responsible for physiological bone resorption. A specific organization of their most prominent cytoskeletal structures, podosomes, is crucial for the degradation of mineralized bone matrix. Each podosome is constituted of an F-actin-enriched central core surrounded by a loose F-actin network, called the podosome cloud. In addition to intrinsic actin dynamics, podosomes are defined by their adhesion to the extracellular matrix, mainly via core-linking CD44 and cloud-linking integrins. These properties allow podosomes to collectively evolve into different patterns implicated in migration and bone resorption. Indeed, to resorb bone, osteoclasts polarize, actively secrete protons, and proteases into the resorption pit where these molecules are confined by a podosome-containing sealing zone. Here, we review recent advancements on podosome structure and regulatory pathways in osteoclasts. We also discuss the distinct functions of different podosome patterns during the lifespan of a single osteoclast.
Keywords: actin; actin rings; bone degradation; migration; osteoclasts; podosomes; sealing zone.
Figures


References
-
- Bartl R. Osteoporosis: diagnosis, prevention, therapy. New York: Springer, 2009.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
