Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech
- PMID: 24714837
- PMCID: PMC3971356
- DOI: 10.6061/clinics/2014(04)10
Gonadotropin therapy in assisted reproduction: an evolutionary perspective from biologics to biotech
Abstract
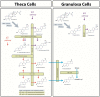
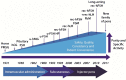
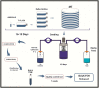
Gonadotropin therapy plays an integral role in ovarian stimulation for infertility treatments. Efforts have been made over the last century to improve gonadotropin preparations. Undoubtedly, current gonadotropins have better quality and safety profiles as well as clinical efficacy than earlier ones. A major achievement has been introducing recombinant technology in the manufacturing processes for follicle-stimulating hormone, luteinizing hormone, and human chorionic gonadotropin. Recombinant gonadotropins are purer than urine-derived gonadotropins, and incorporating vial filling by mass virtually eliminated batch-to-batch variations and enabled accurate dosing. Recombinant and fill-by-mass technologies have been the driving forces for launching of prefilled pen devices for more patient-friendly ovarian stimulation. The most recent developments include the fixed combination of follitropin alfa + lutropin alfa, long-acting FSH gonadotropin, and a new family of prefilled pen injector devices for administration of recombinant gonadotropins. The next step would be the production of orally bioactive molecules with selective follicle-stimulating hormone and luteinizing hormone activity.
Conflict of interest statement
No potential conflict of interest was reported.
Figures

References
-
- Lunenfeld B. Historical perspectives in gonadotrophin therapy. Human Reprod Update. 2004;10(6):453–67. - PubMed
-
- Practice Committee of American Society for Reproductive Medicine, Birmingham, Alabama. Gonadotropin preparations: past, present, and future perspectives. Fertil Steril. 2008;90(5 Suppl):S13–20. - PubMed
-
- Trounson AO, Leeton JF, Wood C, Webb J, Wood J. Pregnancies in humans by fertilization in vitro and embryo transfer in the controlled ovulatory cycle. Science. 1981;212:681–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

