Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes
- PMID: 24715691
- PMCID: PMC4184251
- DOI: 10.1002/stem.1619
Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes
Abstract
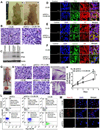
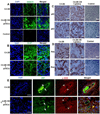
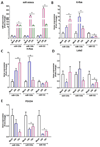
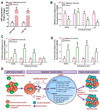
Emerging evidence suggests that mesenchymal stem cells (MSCs) are often recruited to tumor sites but their functional significance in tumor growth and disease progression remains elusive. Herein we report that prostate cancer (PC) cell microenvironment subverts PC patient adipose-derived stem cells (pASCs) to undergo neoplastic transformation. Unlike normal ASCs, the pASCs primed with PC cell conditioned media (CM) formed prostate-like neoplastic lesions in vivo and reproduced aggressive tumors in secondary recipients. The pASC tumors acquired cytogenetic aberrations and mesenchymal-to-epithelial transition and expressed epithelial, neoplastic, and vasculogenic markers reminiscent of molecular features of PC tumor xenografts. Our mechanistic studies revealed that PC cell-derived exosomes are sufficient to recapitulate formation of prostate tumorigenic mimicry generated by CM-primed pASCs in vivo. In addition to downregulation of the large tumor suppressor homolog2 and the programmed cell death protein 4, a neoplastic transformation inhibitor, the tumorigenic reprogramming of pASCs was associated with trafficking by PC cell-derived exosomes of oncogenic factors, including H-ras and K-ras transcripts, oncomiRNAs miR-125b, miR-130b, and miR-155 as well as the Ras superfamily of GTPases Rab1a, Rab1b, and Rab11a. Our findings implicate a new role for PC cell-derived exosomes in clonal expansion of tumors through neoplastic reprogramming of tumor tropic ASCs in cancer patients.
Keywords: Exosomes; OncomiRNAs; Patient adipose derived stem cells; Prostate cancer; Tumor mimicry.
© 2013 AlphaMed Press.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–240. - PubMed
-
- Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

