The nature and extent of contributions by defective ribosome products to the HLA peptidome
- PMID: 24715725
- PMCID: PMC4000780
- DOI: 10.1073/pnas.1321902111
The nature and extent of contributions by defective ribosome products to the HLA peptidome
Abstract
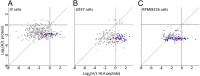
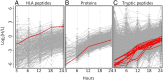
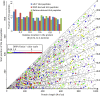
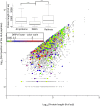
MHC class I peptides are products of endogenous cellular protein degradation. Their prompt presentation, after rapid degradation of their newly synthesized source proteins, is needed to alert the immune system during pathogen infection. A possible source for such rapidly degrading proteins can be defective ribosome products (DRiPs), which include polypeptides produced as part of the pioneer round of translation, premature translation termination, and proteins failing to fold properly or to assemble into their multisubunit protein complexes. However, the identities and relative contribution to the MHC peptidome of these mature or newly synthesized and rapidly degraded cellular proteins is not well understood. To clarify these issues, we used dynamic stable isotope labeling by amino acids in cell culture to define the relative rates of synthesis of the HLA class I peptidomes and the source proteomes of three cultured human hematopoietic cell lines. Large numbers of HLA class I peptides were observed to be derived from DRiPs, defined here as HLA peptides that shift from their light to heavy isotope forms faster than their source proteins. Specific groups of proteins, such as ribosomal and T-complex protein 1 (TCP-1), contributed a disproportionately large number of DRiPs to the HLA peptidomes. Furthermore, no significant preference was observed for HLA peptides derived from the amino terminal regions of the proteins, suggesting that the contribution of products of premature translation termination was minimal. Thus, the most likely sources of DRiPs-derived HLA peptides are full-sized, misassembled, and surplus subunits of large protein complexes.
Keywords: DRiPome; dynamic SILAC; immunopeptidome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Yewdell JW, Antón LC, Bennink JR. Defective ribosomal products (DRiPs): A major source of antigenic peptides for MHC class I molecules? J Immunol. 1996;157(5):1823–1826. - PubMed
-
- Reits EA, Vos JC, Grommé M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404(6779):774–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

