Meta-Review of CSF Core Biomarkers in Alzheimer's Disease: The State-of-the-Art after the New Revised Diagnostic Criteria
- PMID: 24715863
- PMCID: PMC3970033
- DOI: 10.3389/fnagi.2014.00047
Meta-Review of CSF Core Biomarkers in Alzheimer's Disease: The State-of-the-Art after the New Revised Diagnostic Criteria
Abstract
Background: Current research criteria for Alzheimer's disease (AD) include cerebrospinal fluid (CSF) biomarkers into the diagnostic algorithm. However, spreading their use to the clinical routine is still questionable.
Objective: To provide an updated, systematic and critical review on the diagnostic utility of the CSF core biomarkers for AD.
Data sources: MEDLINE, PreMedline, EMBASE, PsycInfo, CINAHL, Cochrane Library, and CRD.
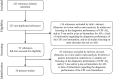
Eligibility criteria: (1a) Systematic reviews with meta-analysis; (1b) Primary studies published after the new revised diagnostic criteria; (2) Evaluation of the diagnostic performance of at least one CSF core biomarker.
Results: The diagnostic performance of CSF biomarkers is generally satisfactory. They are optimal for discriminating AD patients from healthy controls. Their combination may also be suitable for mild cognitive impairment (MCI) prognosis. However, CSF biomarkers fail to distinguish AD from other forms of dementia.
Limitations: (1) Use of clinical diagnosis as standard instead of pathological postmortem confirmation; (2) variability of methodological aspects; (3) insufficiently long follow-up periods in MCI studies; and (4) lower diagnostic accuracy in primary care compared with memory clinics.
Conclusion: Additional work needs to be done to validate the application of CSF core biomarkers as they are proposed in the new revised diagnostic criteria. The use of CSF core biomarkers in clinical routine is more likely if these limitations are overcome. Early diagnosis is going to be of utmost importance when effective pharmacological treatment will be available and the CSF core biomarkers can also be implemented in clinical trials for drug development.
Keywords: Alzheimer’s disease; amyloid beta-protein (42); cerebrospinal fluid biomarkers; meta-review; sensitivity; specificity; state-of-the-art review; tau protein.
Figures
References
-
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement. 7, 270–279 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
-
- Andreasen N., Vanmechelen E., Vanderstichele H., Davidssson P., Vanmechelen E., Davidsson P., et al. (2003). Cerebrospinal fluid levels of total-tau, phospho-tau and Ab42 predicts development of Alzheimer’s disease in patients with mild cognitive impairment. Acta Neurol. Scand. 107(Suppl. 179), 47–51 10.1034/j.1600-0404.107.s179.9.x - DOI - PubMed
-
- Baldeiras I., Santana I., Garrucho M. H., Pascoal R., Lemos R., Santiago B., et al. (2012). CSF biomarkers for the early diagnosis of Alzheimer’s disease in a routine clinical setting – the first Portuguese study. Sinapse 12, 14–22
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources