Risk factors for physical impairment after acute lung injury in a national, multicenter study
- PMID: 24716641
- PMCID: PMC4061900
- DOI: 10.1164/rccm.201401-0158OC
Risk factors for physical impairment after acute lung injury in a national, multicenter study
Abstract
Rationale: Existing studies of risk factors for physical impairments in acute lung injury (ALI) survivors were potentially limited by single-center design or relatively small sample size.
Objectives: To evaluate risk factors for three measures of physical impairments commonly experienced by survivors of ALI in the first year after hospitalization.
Methods: A prospective, longitudinal study of 6- and 12-month physical outcomes (muscle strength, 6-minute-walk distance, and Short Form [SF]-36 Physical Function score) for 203 survivors of ALI enrolled from 12 hospitals participating in the ARDS Network randomized trials. Multivariable regression analyses evaluated the independent association of critical illness-related variables and intensive care interventions with impairments in each physical outcome measure, after adjusting for patient demographics, comorbidities, and baseline functional status.
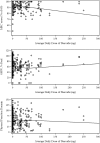
Measurements and main results: At 6 and 12 months, respectively, mean (± SD) values for strength (presented as proportion of maximum strength score evaluated using manual muscle testing) was 92% (± 8%) and 93% (± 9%), 6-minute-walk distance (as percent-predicted) was 64% (± 22%) and 67% (± 26%), and SF-36 Physical Function score (as percent-predicted) was 61% (± 36%) and 67% (± 37%). After accounting for patient baseline status, there was significant association and statistical interaction of mean daily dose of corticosteroids and intensive care unit length of stay with impairments in physical outcomes.
Conclusions: Patients had substantial impairments, from predicted values, for 6-minute-walk distance and SF-36 Physical Function outcome measures. Minimizing corticosteroid dose and implementing existing evidence-based methods to reduce duration of intensive care unit stay and associated patient immobilization may be important interventions for improving ALI survivors' physical outcomes.
Figures
References
-
- Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. - PubMed
-
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL096504/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- HHSN268200536171C/PHS HHS/United States
- UL1 TR 000424-06/TR/NCATS NIH HHS/United States
- HHSN268200536174C/PHS HHS/United States
- R01HL091760-02S1/HL/NHLBI NIH HHS/United States
- UL1 TR000424/TR/NCATS NIH HHS/United States
- HHSN268200536173C/PHS HHS/United States
- HSN268200536175C/PHS HHS/United States
- HSN268200536170C/PHS HHS/United States
- R01 HL091760/HL/NHLBI NIH HHS/United States
- HHSN268200536179C/PHS HHS/United States
- R01HL091760/HL/NHLBI NIH HHS/United States
- R01HL096504/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

