Glycosylation, hypogammaglobulinemia, and resistance to viral infections
- PMID: 24716661
- PMCID: PMC4066413
- DOI: 10.1056/NEJMoa1302846
Glycosylation, hypogammaglobulinemia, and resistance to viral infections
Abstract
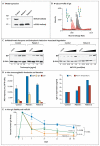
Genetic defects in MOGS, the gene encoding mannosyl-oligosaccharide glucosidase (the first enzyme in the processing pathway of N-linked oligosaccharide), cause the rare congenital disorder of glycosylation type IIb (CDG-IIb), also known as MOGS-CDG. MOGS is expressed in the endoplasmic reticulum and is involved in the trimming of N-glycans. We evaluated two siblings with CDG-IIb who presented with multiple neurologic complications and a paradoxical immunologic phenotype characterized by severe hypogammaglobulinemia but limited clinical evidence of an infectious diathesis. A shortened immunoglobulin half-life was determined to be the mechanism underlying the hypogammaglobulinemia. Impaired viral replication and cellular entry may explain a decreased susceptibility to infections.
Figures

Comment in
-
Viral resistance of MOGS-CDG patients implies a broad-spectrum strategy against acute virus infections.Antivir Ther. 2015;20(3):257-9. doi: 10.3851/IMP2907. Epub 2014 Oct 15. Antivir Ther. 2015. PMID: 25318123 Free PMC article.
References
-
- Varki A. Essentials of glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2009. - PubMed
-
- Faid V, Chirat F, Seta N, Foulquier F, Morelle W. A rapid mass spectrometric strategy for the characterization of N- and O-glycan chains in the diagnosis of defects in glycan biosynthesis. Proteomics. 2007;7:1800–13. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
