Variation in motor output and motor performance in a centrally generated motor pattern
- PMID: 24717348
- PMCID: PMC4064392
- DOI: 10.1152/jn.00856.2013
Variation in motor output and motor performance in a centrally generated motor pattern
Abstract
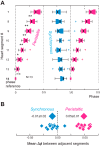
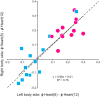
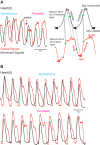
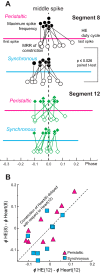
Central pattern generators (CPGs) produce motor patterns that ultimately drive motor outputs. We studied how functional motor performance is achieved, specifically, whether the variation seen in motor patterns is reflected in motor performance and whether fictive motor patterns differ from those in vivo. We used the leech heartbeat system in which a bilaterally symmetrical CPG coordinates segmental heart motor neurons and two segmented heart tubes into two mutually exclusive coordination modes: rear-to-front peristaltic on one side and nearly synchronous on the other, with regular side-to-side switches. We assessed individual variability of the motor pattern and the beat pattern in vivo. To quantify the beat pattern we imaged intact adults. To quantify the phase relations between motor neurons and heart constrictions we recorded extracellularly from two heart motor neurons and movement from the corresponding heart segments in minimally dissected leeches. Variation in the motor pattern was reflected in motor performance only in the peristaltic mode, where larger intersegmental phase differences in the motor neurons resulted in larger phase differences between heart constrictions. Fictive motor patterns differed from those in vivo only in the synchronous mode, where intersegmental phase differences in vivo had a larger front-to-rear bias and were more constrained. Additionally, load-influenced constriction timing might explain the amplification of the phase differences between heart segments in the peristaltic mode and the higher variability in motor output due to body shape assumed in this soft-bodied animal. The motor pattern determines the beat pattern, peristaltic or synchronous, but heart mechanics influence the phase relations achieved.
Keywords: intersegmental coordination; leech; motor pattern in vivo; motor performance; variability.
Copyright © 2014 the American Physiological Society.
Figures








References
-
- Arbas EA. Rate modification in the heartbeat central pattern generator of the medicinal leech. J Comp Physiol A 155: 783–794, 1984
-
- Arbas EA, Calabrese RL. Leydig neuron activity modulates heartbeat in the medicinal leech. J Comp Physiol A 167: 665–671, 1990 - PubMed
-
- Brezina V, Orekhova IV, Weiss KR. The neuromuscular transform: the dynamic, nonlinear link between motor neuron firing patterns and muscle contraction in rhythmic behaviors. J Neurophysiol 83: 207–231, 2000 - PubMed
-
- Calabrese RL. The neural control of alternate heartbeat coordination states in the leech, Hirudo medicinalis. J Comp Physiol A 122: 11–143, 1977
-
- Calabrese RL. The roles of endogenous membrane properties and synaptic interaction in generating the heartbeat rhythm of the leech, Hirudo medicinalis. J Exp Biol 82: 163–176, 1979 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

