Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion
- PMID: 24717428
- PMCID: PMC4006097
- DOI: 10.1038/nature13242
Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion
Abstract
Entamoeba histolytica is the causative agent of amoebiasis, a potentially fatal diarrhoeal disease in the developing world. The parasite was named "histolytica" for its ability to destroy host tissues, which is probably driven by direct killing of human cells. The mechanism of human cell killing has been unclear, although the accepted model was that the parasites use secreted toxic effectors to kill cells before ingestion. Here we report the discovery that amoebae kill by ingesting distinct pieces of living human cells, resulting in intracellular calcium elevation and eventual cell death. After cell killing, amoebae detach and cease ingestion. Ingestion of human cell fragments is required for cell killing, and also contributes to invasion of intestinal tissue. The internalization of fragments of living human cells is reminiscent of trogocytosis (from Greek trogo, nibble) observed between immune cells, but amoebic trogocytosis differs because it results in death. The ingestion of live cell material and the rejection of corpses illuminate a stark contrast to the established model of dead cell clearance in multicellular organisms. These findings change the model for tissue destruction in amoebiasis and suggest an ancient origin of trogocytosis as a form of intercellular exchange.
Conflict of interest statement
The authors declare that no competing financial interests exist.
Figures


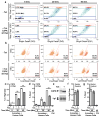
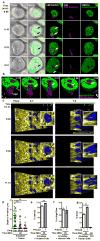
Comment in
-
Infection biology: Nibbled to death.Nature. 2014 Apr 24;508(7497):462-3. doi: 10.1038/nature13223. Epub 2014 Apr 9. Nature. 2014. PMID: 24717438 No abstract available.
-
Tissue destruction in amebiasis: chew on it.Clin Infect Dis. 2014 Jul 15;59(2):iii-iv. Clin Infect Dis. 2014. PMID: 25105188 No abstract available.
References
-
- Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. - PubMed
-
- Huang JF, et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–954. - PubMed
-
- Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166:3645–3649. - PubMed
-
- Hudson L, Sprent J, Miller JF, Playfair JH. B cell-derived immunoglobulin on activated mouse T lymphocytes. Nature. 1974;251:60–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

