Piezo2 is required for Merkel-cell mechanotransduction
- PMID: 24717433
- PMCID: PMC4039622
- DOI: 10.1038/nature13251
Piezo2 is required for Merkel-cell mechanotransduction
Abstract
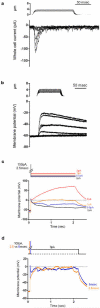
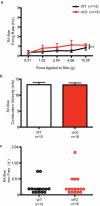
How we sense touch remains fundamentally unknown. The Merkel cell-neurite complex is a gentle touch receptor in the skin that mediates slowly adapting responses of Aβ sensory fibres to encode fine details of objects. This mechanoreceptor complex was recognized to have an essential role in sensing gentle touch nearly 50 years ago. However, whether Merkel cells or afferent fibres themselves sense mechanical force is still debated, and the molecular mechanism of mechanotransduction is unknown. Synapse-like junctions are observed between Merkel cells and associated afferents, and yet it is unclear whether Merkel cells are inherently mechanosensitive or whether they can rapidly transmit such information to the neighbouring nerve. Here we show that Merkel cells produce touch-sensitive currents in vitro. Piezo2, a mechanically activated cation channel, is expressed in Merkel cells. We engineered mice deficient in Piezo2 in the skin, but not in sensory neurons, and show that Merkel-cell mechanosensitivity completely depends on Piezo2. In these mice, slowly adapting responses in vivo mediated by the Merkel cell-neurite complex show reduced static firing rates, and moreover, the mice display moderately decreased behavioural responses to gentle touch. Our results indicate that Piezo2 is the Merkel-cell mechanotransduction channel and provide the first line of evidence that Piezo channels have a physiological role in mechanosensation in mammals. Furthermore, our data present evidence for a two-receptor-site model, in which both Merkel cells and innervating afferents act together as mechanosensors. The two-receptor system could provide this mechanoreceptor complex with a tuning mechanism to achieve highly sophisticated responses to a given mechanical stimulus.
Figures
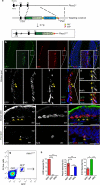
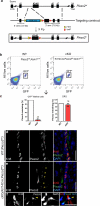
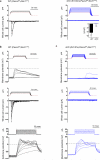
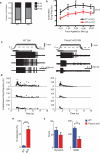


Comment in
-
Sensory transduction: Merkel cells bring a delicate touch.Nat Rev Neurosci. 2014 Jun;15(6):348-9. doi: 10.1038/nrn3748. Epub 2014 Apr 25. Nat Rev Neurosci. 2014. PMID: 24762930 No abstract available.
References
-
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Current opinion in neurobiology. 2001;11:455–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

