Enantioselective construction of remote quaternary stereocentres
- PMID: 24717439
- PMCID: PMC4007023
- DOI: 10.1038/nature13231
Enantioselective construction of remote quaternary stereocentres
Abstract
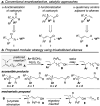
Small molecules that contain all-carbon quaternary stereocentres-carbon atoms bonded to four distinct carbon substituents-are found in many secondary metabolites and some pharmaceutical agents. The construction of such compounds in an enantioselective fashion remains a long-standing challenge to synthetic organic chemists. In particular, methods for synthesizing quaternary stereocentres that are remote from other functional groups are underdeveloped. Here we report a catalytic and enantioselective intermolecular Heck-type reaction of trisubstituted-alkenyl alcohols with aryl boronic acids. This method provides direct access to quaternary all-carbon-substituted β-, γ-, δ-, ε- or ζ-aryl carbonyl compounds, because the unsaturation of the alkene is relayed to the alcohol, resulting in the formation of a carbonyl group. The scope of the process also includes incorporation of pre-existing stereocentres along the alkyl chain, which links the alkene and the alcohol, in which the stereocentre is preserved. The method described allows access to diverse molecular building blocks containing an enantiomerically enriched quaternary centre.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Comment in
-
Organic chemistry: Catalysis marches on.Nature. 2014 Apr 17;508(7496):324-5. doi: 10.1038/nature13225. Epub 2014 Apr 9. Nature. 2014. PMID: 24717440 No abstract available.
References
-
- Trost BM, Jiang C. Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis. 2006:369–396.
-
- Christoffers J, Mann A. Enantioselective construction of quaternary stereocenters. Angew Chem Int Ed. 2001;40:4591–4597. - PubMed
-
- Das JP, Marek I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem Commun. 2011;47:4593–4623. - PubMed
-
- Marek I, et al. All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C–C bonds per chemical step. J Am Chem Soc. 2014;136:2682–2694. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

