Controlling the structural and functional anisotropy of engineered cardiac tissues
- PMID: 24717534
- PMCID: PMC4040155
- DOI: 10.1088/1758-5082/6/2/024109
Controlling the structural and functional anisotropy of engineered cardiac tissues
Abstract
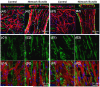
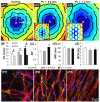
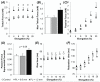
The ability to control the degree of structural and functional anisotropy in 3D engineered cardiac tissues would have high utility for both in vitro studies of cardiac muscle physiology and pathology as well as potential tissue engineering therapies for myocardial infarction. Here, we applied a high aspect ratio soft lithography technique to generate network-like tissue patches seeded with neonatal rat cardiomyocytes. Fabricating longer elliptical pores within the patch networks increased the overall cardiomyocyte and extracellular matrix alignment within the patch. Improved uniformity of cell and matrix alignment yielded an increase in anisotropy of action potential propagation and faster longitudinal conduction velocity (LCV). Cardiac tissue patches with a higher degree of cardiomyocyte alignment and electrical anisotropy also demonstrated greater isometric twitch forces. After two weeks of culture, specific measures of electrical and contractile function (LCV = 26.8 ± 0.8 cm s(-1), specific twitch force = 8.9 ± 1.1 mN mm(-2) for the longest pores studied) were comparable to those of neonatal rat myocardium. We have thus described methodology for engineering of highly functional 3D engineered cardiac tissues with controllable degree of anisotropy.
Figures


References
-
- Donndorf P, Strauer BE, Haverich A, Steinhoff G. Stem cell therapy for the treatment of acute myocardial infarction and chronic ischemic heart disease. Current Pharmaceutical Biotechnology. 2013;14:12–19. - PubMed
-
- Delewi R, Andriessen A, Tijssen JGP, Zijlstra F, Piek JJ, Hirsch A. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A meta-analysis of randomised controlled clinical trials. Heart. 2013;99:225–232. - PubMed
-
- King A. Stem cells. Could it be time to abandon BMCs? Nature Reviews - Cardiology. 2013;10:8. - PubMed
-
- Marbán E, Malliaras K. Mixed results for bone marrow–derived cell therapy for ischemic heart disease. Journal of the American Medical Association. 2012;308:2405–2406. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
