3D printing facilitated scaffold-free tissue unit fabrication
- PMID: 24717646
- PMCID: PMC4418504
- DOI: 10.1088/1758-5082/6/2/024111
3D printing facilitated scaffold-free tissue unit fabrication
Abstract
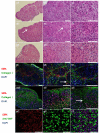
Tissue spheroids hold great potential in tissue engineering as building blocks to assemble into functional tissues. To date, agarose molds have been extensively used to facilitate fusion process of tissue spheroids. As a molding material, agarose typically requires low temperature plates for gelation and/or heated dispenser units. Here, we proposed and developed an alginate-based, direct 3D mold-printing technology: 3D printing microdroplets of alginate solution into biocompatible, bio-inert alginate hydrogel molds for the fabrication of scaffold-free tissue engineering constructs. Specifically, we developed a 3D printing technology to deposit microdroplets of alginate solution on calcium containing substrates in a layer-by-layer fashion to prepare ring-shaped 3D hydrogel molds. Tissue spheroids composed of 50% endothelial cells and 50% smooth muscle cells were robotically placed into the 3D printed alginate molds using a 3D printer, and were found to rapidly fuse into toroid-shaped tissue units. Histological and immunofluorescence analysis indicated that the cells secreted collagen type I playing a critical role in promoting cell-cell adhesion, tissue formation and maturation.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
