Targeting class IA PI3K isoforms selectively impairs cell growth, survival, and migration in glioblastoma
- PMID: 24718026
- PMCID: PMC3981776
- DOI: 10.1371/journal.pone.0094132
Targeting class IA PI3K isoforms selectively impairs cell growth, survival, and migration in glioblastoma
Abstract
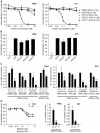
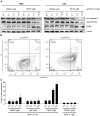
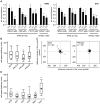
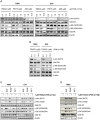
The phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway is frequently activated in human cancer and plays a crucial role in glioblastoma biology. We were interested in gaining further insight into the potential of targeting PI3K isoforms as a novel anti-tumor approach in glioblastoma. Consistent expression of the PI3K catalytic isoform PI3K p110α was detected in a panel of glioblastoma patient samples. In contrast, PI3K p110β expression was only rarely detected in glioblastoma patient samples. The expression of a module comprising the epidermal growth factor receptor (EGFR)/PI3K p110α/phosphorylated ribosomal S6 protein (p-S6) was correlated with shorter patient survival. Inhibition of PI3K p110α activity impaired the anchorage-dependent growth of glioblastoma cells and induced tumor regression in vivo. Inhibition of PI3K p110α or PI3K p110β also led to impaired anchorage-independent growth, a decreased migratory capacity of glioblastoma cells, and reduced the activation of the Akt/mTOR pathway. These effects were selective, because targeting of PI3K p110δ did not result in a comparable impairment of glioblastoma tumorigenic properties. Together, our data reveal that drugs targeting PI3K p110α can reduce growth in a subset of glioblastoma tumors characterized by the expression of EGFR/PI3K p110α/p-S6.
Conflict of interest statement
Figures


References
-
- Central Brain Tumor Registry of the United States CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–22007.
-
- Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359: 492–507. - PubMed
-
- DeAngelis LM (2001) Brain tumors. N Engl J Med 344: 114–123. - PubMed
-
- Halatsch ME, Schmidt U, Behnke-Mursch J, Unterberg A, Wirtz CR (2006) Epidermal growth factor receptor inhibition for the treatment of glioblastoma multiforme and other malignant brain tumours. Cancer Treat Rev 32: 74–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

