Correlated optical and isotopic nanoscopy
- PMID: 24718107
- PMCID: PMC3996535
- DOI: 10.1038/ncomms4664
Correlated optical and isotopic nanoscopy
Abstract
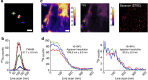
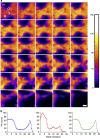
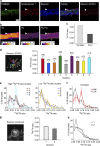
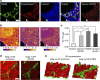
The isotopic composition of different materials can be imaged by secondary ion mass spectrometry. In biology, this method is mainly used to study cellular metabolism and turnover, by pulsing the cells with marker molecules such as amino acids labelled with stable isotopes ((15)N, (13)C). The incorporation of the markers is then imaged with a lateral resolution that can surpass 100 nm. However, secondary ion mass spectrometry cannot identify specific subcellular structures like organelles, and needs to be correlated with a second technique, such as fluorescence imaging. Here, we present a method based on stimulated emission depletion microscopy that provides correlated optical and isotopic nanoscopy (COIN) images. We use this approach to study the protein turnover in different organelles from cultured hippocampal neurons. Correlated optical and isotopic nanoscopy can be applied to a variety of biological samples, and should therefore enable the investigation of the isotopic composition of many organelles and subcellular structures.
Conflict of interest statement
François Hillion is an employee of Cameca, a company manufacturing SIMS instruments. All the other authors declare no competing financial interests.
Figures
References
-
- Johansson M. E. V. & Hansson G. C. Analysis of assembly of secreted mucins. Methods. Mol. Biol. 842, 109–121 (2012). - PubMed
-
- Dittrich A., Siewert E. & Schaper F. Determination of protein turnover rates in the Jak/STAT pathway using a radioactive pulse-chase approach. Methods. Mol. Biol. 967, 69–80 (2013). - PubMed
-
- Zhang J., Campbell R. E., Ting A. Y. & Tsien R. Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell. Biol. 3, 907–918 (2002). - PubMed
-
- Zhang L. et al. Method for real-time monitoring of protein degradation at the single cell level. Biotechniques 42, 446–450 (2007). - PubMed
-
- Hindie E., Coulomb B., Beaupain R. & Galle P. Mapping the cellular distribution of labelled molecules by SIMS microscopy. Biol. Cell. 74, 81–88 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

