The CB1 receptor as an important mediator of hedonic reward processing
- PMID: 24718372
- PMCID: PMC4138748
- DOI: 10.1038/npp.2014.86
The CB1 receptor as an important mediator of hedonic reward processing
Abstract
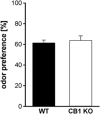
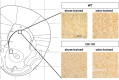
The endocannabinoid (ECB) system has emerged recently as a key mediator for reward processing. It is well known that cannabinoids affect appetitive learning processes and can induce reinforcing and rewarding effects. However, the involvement of the ECB system in hedonic aspects of reward-related behavior is not completely understood. With the present study, we investigated the modulatory role of the ECB system on hedonic perception, measured by the pleasure attenuated startle (PAS) paradigm for a palatable food reward. Here, a conditioned odor is thought to induce a pleasant affective state that attenuates an aversive reflex-the acoustic startle response. Modulatory effects of the CB1 receptor antagonist/inverse agonist SR1411716 and the cannabinoid agonist WIN 55 212-2 on PAS were examined in rats. PAS was also measured in CB1 receptor knockout (KO) and wild-type (WT) mice. Pharmacological inhibition as well as the absence of CB1 receptors was found to reduce PAS, whereas WIN 55 212-2 administration increased PAS. Finally, presentation of a conditioned reward cue was found to induce striatal FosB/ΔFosB expression in WT mice, but not in KO mice, indicating a reduced stimulation of reward-related brain regions in conditioned KO mice by odor presentation. We here show that in addition to our previous studies in rats, PAS may also serve as a valuable and suitable measure to assess hedonic processing in mice. Our data further indicate that the ECB system, and in particular CB1 receptor signaling, appears to be highly important for the mediation of hedonic aspects of reward processing.
Figures



References
-
- Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. - PubMed
-
- Arnold JC, Topple AN, Mallet PE, Hunt GE, McGregor IS. The distribution of cannabinoid-induced Fos expression in rat brain: differences between the Lewis and Wistar strain. Brain Res. 2001;921:240–255. - PubMed
-
- Arnone M, Maruani J, Chaperon F, Thiebot M, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR 1411716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. - PubMed
-
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

