Locomotor function shapes the passive mechanical properties and operating lengths of muscle
- PMID: 24718759
- PMCID: PMC3996596
- DOI: 10.1098/rspb.2013.2914
Locomotor function shapes the passive mechanical properties and operating lengths of muscle
Abstract
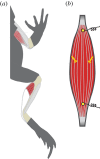
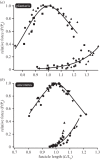
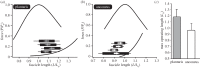
Locomotor muscles often perform diverse roles, functioning as motors that produce mechanical energy, struts that produce force and brakes that dissipate mechanical energy. In many vertebrate muscles, these functions are not mutually exclusive and a single muscle often performs a range of mechanically diverse tasks. This functional diversity has obscured the relationship between a muscle's locomotor function and its mechanical properties. I use hopping in toads as a model system for comparing muscles that primarily produce mechanical energy with muscles that primarily dissipate mechanical energy. During hopping, hindlimb muscles undergo active shortening to produce mechanical energy and propel the animal into the air, whereas the forelimb muscles undergo active lengthening to dissipate mechanical energy during landing. Muscles performing distinct mechanical functions operate on different regions of the force-length curve. These findings suggest that a muscle's operating length may be shaped by potential trade-offs between force production and sarcomere stability. In addition, the passive force-length properties of hindlimb and forelimb muscles vary, suggesting that passive stiffness functions to restrict the muscle's operating length in vivo. These results inform our understanding of vertebrate muscle variation by providing a clear link between a muscle's locomotor function and its mechanical properties.
Keywords: force–length; hopping; landing; length–tension; muscle stiffness; passive elasticity.
Figures


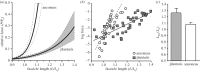
References
-
- Dickinson MH, Farley CT, Full RJ, Koehl MAR, Kram R, Lehman S. 2000. How animals move: an integrative view. Science 288, 100–106 (doi:10.1126/science.288.5463.100) - DOI - PubMed
-
- Daley MA, Biewener AA. 2003. Muscle force–length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 206, 2941–2958 (doi:10.1242/jeb.00503) - DOI - PubMed
-
- Ahn AN, Meijer K, Full RJ. 2006. In situ muscle power differs without varying in vitro mechanical properties in two insect leg muscles innervated by the same motor neuron. J. Exp. Biol. 209, 3370–3382 (doi:10.1242/jeb.02392) - DOI - PubMed
-
- Swoap SJ, Johnson TP, Josephson RK, Bennett AF. 1993. Temperature, muscle power output and limitations on burst locomotor performance of the lizard Dipsosaurus dorsalis. J. Exp. Biol. 174, 185–197
-
- George NT, Sponberg S, Daniel TL. 2012. Temperature gradients drive mechanical energy gradients in the flight muscle of Manduca sexta. J. Exp. Biol. 215, 471–479 (doi:10.1242/jeb.062901) - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
